Abstract
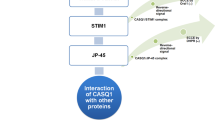
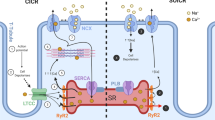
Calsequestrin (CASQ) is the most abundant Ca2+ binding protein localized in the sarcoplasmic reticulum (SR) of skeletal and cardiac muscle. The genome of vertebrates contains two genes, CASQ1 and CASQ2. CASQ1 and CASQ2 have a high level of homology, but show specific patterns of expression. Fast-twitch skeletal muscle fibers express only CASQ1, both CASQ1 and CASQ2 are present in slow-twitch skeletal muscle fibers, while CASQ2 is the only protein present in cardiomyocytes. Depending on the intraluminal SR Ca2+ levels, CASQ monomers assemble to form large polymers, which increase their Ca2+ binding ability. CASQ interacts with triadin and junctin, two additional SR proteins which contribute to localize CASQ to the junctional region of the SR (j-SR) and also modulate CASQ ability to polymerize into large macromolecular complexes. In addition to its ability to bind Ca2+ in the SR, CASQ appears also to be able to contribute to regulation of Ca2+ homeostasis in muscle cells. Both CASQ1 and CASQ2 are able to either activate and inhibit the ryanodine receptors (RyRs) calcium release channels, likely through their interactions with junctin and triadin. Additional evidence indicates that CASQ1 contributes to regulate the mechanism of store operated calcium entry in skeletal muscle via a direct interaction with the Stromal Interaction Molecule 1 (STIM1). Mutations in CASQ2 and CASQ1 have been identified, respectively, in patients with catecholamine-induced polymorphic ventricular tachycardia and in patients with some forms of myopathy. This review will highlight recent developments in understanding CASQ1 and CASQ2 in health and diseases.

Similar content being viewed by others
References
Bal NC, Sharon A, Gupta SC, Jena N, Shaikh S, Gyorke S, Periasamy M (2010) The catecholaminergic polymorphic ventricular tachycardia mutation R33Q disrupts the N-terminal structural motif that regulates reversible calsequestrin polymerization. J Biol Chem 28(22):17188–17196. https://doi.org/10.1074/jbc.M109.096354
Bal NC, Jena N, Chakravarty H, Kumar A, Chi M, Balaraju T, Rawale SV, Rawale JS, Sharon A, Periasamy M (2015) The C-terminal calcium-sensitive disordered motifs regulate isoform-specific polymerization characteristics of calsequestrin. Biopolymers 103(1):15–22. https://doi.org/10.1002/bip.22534
Barone V, Del Re V, Gamberucci A, Polverino V, Galli L, Rossi D, Costanzi E, Toniolo L, Berti G, Malandrini A, Ricci G, Siciliano G, Vattemi G, Tomelleri G, Pierantozzi E, Spinozzi S, Volpi N, Fulceri R, Battistutta R, Reggiani C, Sorrentino V (2017) Identification and characterization of three novel mutations in the CASQ1 gene in four patients with tubular aggregate myopathy. Hum Mutat 38(12):1761–1773. https://doi.org/10.1002/humu.23338
Beard NA, Sakowska MM, Dulhunty AF, Laver DR (2002) Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys J 82(1 Pt 1):310–320
Beard NA, Casarotto MG, Wei L, Varsányi M, Laver DR, Dulhunty AF (2005) Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J 88(5):3444–3454
Beard NA, Wei L, Cheung SN, Kimura T, Varsányi M, Dulhunty AF (2008) Phosphorylation of skeletal muscle calsequestrin enhances its Ca2+ binding capacity and promotes its association with junctin. Cell Calcium 44(4):363–373
Beard NA, Dulhunty AF (2015) C-terminal residues of skeletal muscle calsequestrin are essential for calcium binding and for skeletal ryanodine receptor inhibition. Skelet Muscle 22:5:6. https://doi.org/10.1186/s13395-015-0029-7
Beltrán M, Barrientos G, Hidalgo C (2006) Fast kinetics of calcium dissociation from calsequestrin. Biol Res 39(3):493–503
Bohm J, Chevessier F, Maues De Paula A, Koch C, Attarian S, Feger C, Hantaï D, Laforet P, Ghorab K, Vallat JM, Fardeau M, Figarella-Branger D, Pouget J, Romero NB, Koch M, Ebel C, Levy N, Krahn M, Eymard B, Bartoli M, Laporte J (2013) Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am J Hum Genet 7;92(2):271–278
Bohm J, Bulla M, Urquhart JE, Malfatti E, Williams SG, O’Sullivan J, Szlauer A, Koch C, Baranello G, Mora M, Ripolone M, Violano R, Moggio M, Kingston H, Dawson T, DeGoede CG, Nixon J, Boland A, Deleuze JF, Romero N, Newman WG, Demaurex N, Laporte J (2017) ORAI1 mutations with distinct channel gating defects in tubular aggregate myopathy. Hum Mut 38(4):426–438
Bohm J, Laporte J (2018a) Gain-of-function mutations in STIM1 and ORAI1 causing tubular aggregate myopathy and Stormorken syndrome. Cell Calcium 76:1–9
Bohm J, Lornage X, Chevessier F, Birck C, Zanotti S, Cudia P, Bulla M, Granger F, Bui M, Sartori M, Schneider-Gold C, Malfatti E, Romero NB, Mora M, Laporte J (2018b) CASQ1 mutations impair calsequestrin polymerization and cause tubular aggregate myopathy. Acta Neuropathol 135:149–151
Boncompagni S, Thomas M, Lopez JR, Allen PD, Yuan Q, Kranias EG, Franzini-Armstrong C, Perez CF (2012) Triadin/Junctin double null mouse reveals a differential role for Triadin and Junctin in anchoring CASQ to the jSR and regulating Ca(2+) homeostasis. PLoS ONE 7(7):e39962. https://doi.org/10.1371/journal.pone.0039962
Boncompagni S, Michelucci A, Pietrangelo L, Dirksen RT, Protasi F (2017) Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci Rep 7(1):14286
Boncompagni S, Michelucci A, Pietrangelo L, Dirksen RT, Protasi F (2018) Addendum: exercise- dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci Rep 8(1):17463
Bjorksten AR, Gillies RL, Hockey BM, Du Sart D (2016) Sequencing of genes involved in the movement of calcium across human skeletal muscle sarcoplasmic reticulum: continuing the search for genes associated with malignant hyperthermia. Anaesth Intensive Care 44(6):762–768
Cacheux M, Fauconnier J, Thireau J, Osseni A, Brocard J, Roux-Buisson N, Brocard J, Fauré J, Lacampagne A, Marty I (2020) Interplay between triadin and calsequestrin in the pathogenesis of CPVT in the mouse. Mol Ther 28(1):171–179. https://doi.org/10.1016/j.ymthe.2019.09.012
Canato M, Scorzeto M, Giacomello M, Protasi F, Reggiani C, Stienen GJ (2010) Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc Natl Acad Sci USA 107(51):22326–22331. https://doi.org/10.1073/pnas.1009168108
Chen H, Valle G, Furlan S, Nani A, Gyorke S, Fill M, Volpe P (2013) Mechanism of calsequestrin regulation of single cardiac ryanodine receptor in normal and pathological conditions. J Gen Physiol 142(2):127–136. https://doi.org/10.1085/jgp.201311022
Cho MC, Rapacciuolo A, Koch WJ, Kobayashi Y, Jones LR, Rockman HA (1999) Defective beta-adrenergic receptor signaling precedes the development of dilated cardiomyopathy in transgenic mice with calsequestrin overexpression. J Biol Chem 6(32):22251–22256
Cho JH1, Ko KM, Singaruvelu G, Lee W, Kang GB, Rho SH, Park BJ, Yu JR, Kagawa H, Eom SH, Kim DH, Ahnn J (2007) Functional importance of polymerization and localization of calsequestrin in C. elegans. J Cell Sci 120(1):1551–1558
Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, Knollmann BC (2007) Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res 14(6):617–626
D’Adamo MC, Sforna L, Visentin S, Grottesi A, Servettini L, Guglielmi L, Macchioni L, Saredi S, Curcio M, De Nuccio C, Hasan S, Corazzi L, Franciolini F, Mora M, Catacuzzeno L, Pessia M (2016) A calsequestrin-1 mutation associated with a skeletal muscle disease alters sarcoplasmic Ca2+ release. PLoS ONE 11(5):e0155516. https://doi.org/10.1371/journal.pone.0155516
Dainese M, Quarta M, Lyfenko AD, Paolini C, Canato M, Reggiani C, Dirksen RT, Protasi F (2009) Anesthetic- and heat-induced sudden death in calsequestrin-1-knockout mice. FASEB J 23(6):1710–1720. https://doi.org/10.1096/fj.08-121335
Damiani E, Volpe P, Margreth A (1990) Coexpression of two isoforms of calsequestrin in rabbit slow-twitch muscle. J Muscle Res Cell Motil 11(6):522–530
Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L (2011) STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J Cell Biol 25(2):335–346
Das SK, Chu W, Zhang Z, Hasstedt SJ, Elbein SC (2004) Calsquestrin 1 (CASQ1) gene polymorphisms under chromosome 1q21 linkage peak are associated with type 2 diabetes in Northern European Caucasians. Diabetes 53(12):3300–3006
de Haan S, Lahooti H, Morris O, Wall JR (2010) Epitopes, immunoglobulin classes and immunoglobulin G subclasses of calsequestrin antibodies in patients with thyroid eye disease. Autoimmunity 43(8):698–703. https://doi.org/10.3109/08916931003774954
de la Fuente S, Van Langen IM, Postma AV, Bikker H, Meijer A (2008) A case of catecholaminergic polymorphic ventricular tachycardia caused by two calsequestrin 2 mutations. Pacing Clin Electrophysiol 31(7):916–919. https://doi.org/10.1111/j.1540-8159.2008.01111.x
di Barletta MR, Viatchenko-Karpinski S, Nori A, Memmi M, Terentyev D, Turcato F, Valle G, Rizzi N, Napolitano C, Gyorke S, Volpe P, Priori SG (2006) Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation 5(10):1012–1019
Dirksen WP, Lacombe VA, Chi M, Kalyanasundaram A, Viatchenko-Karpinski S, Terentyev D, Zhou Z, Vedamoorthyrao S, Li N, Chiamvimonvat N, Carnes CA, Franzini-Armstrong C, Györke S, Periasamy M (2007) A mutation in calsequestrin, CASQ2D307H, impairs sarcoplasmic reticulum Ca2+ handling and causes complex ventricular arrhythmias in mice. Cardiovasc Res 75(1):69–78
Dulhunty A, Wei L, Beard N (2009) Junctin—the quiet achiever. J Physiol 587:3135–3137
Eldar M, Pras E, Lahat H (2003) A missense mutation in the CASQ2 gene is associated with autosomal-recessive catecholamine-induced polymorphic ventricular tachycardia. Trends Cardiovasc Med 13(4):148–151
Faggioni M, Hwang HS, van der Werf C, Nederend I, Kannankeril PJ, Wilde AAM, Knollmann BC (2013) Accelerated sinus rhythm prevents catecholaminergic polymorphic ventricular tachycardia in mice and in patients. Circ Res 112:689–697
Flores DJ, Duong T, Brandenberger LO, Mitra A, Shiral A, Johnson JC, Springer D, Noguchi A, Y Z, Ebert SN, Ludwig A, Knollmann BC, Levin MD, Pfeifer K (2018) Conditional ablation and conditional rescue models for Casq2 elucidate the role of development and of cell-type specific expression of Casq2 in the CPVT2 phenotype. Hum Mol Gen 27:1533–1544
Franzini-Armstrong C, Kennery LJ, Varriano-Martson E (1987) The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J Cell Biol 105(1):49–56
Fu M, Damcott CM, Sabra M, Pollin TI, Ott SH, Wang J, Garant MJ, O’Connell JR, Mitchell BD, Shuldiner AR (2004) Polymorphism in the calsequestrin 1 (CASQ1) gene on chromosome 1q21 is associated with type 2 diabetes in the old order Amish. Diabetes 53(12):3292–3299
Furlan S, Mosole S, Murgia M, Nagaraj N, Argenton F, Volpe P, Nori A (2016) Calsequestrins in skeletal and cardiac muscle from adult Danio rerio. J Muscle Res Cell Motil 37(1–2):27–39. https://doi.org/10.1007/s10974-015-9432-2.
Fujii J, Willard HF, MacLennan DH (1990) Characterization and localization to human chromosome 1 of human fast-twitch skeletal muscle calsequestrin gene. Somat Cell Mol Genet 16(2):185–189
Fujisawa T, Aizawa Y, Katsumata Y, Udo A, Ito S, Hatakeyama K, Hirose M, Miyama H, Nakajima K, Nishiyama T, Kimura T, Nitta M, Misumi K, Takatsuki S, Kosaki K, Fukuda K (2019) A homozygous CASQ2 mutation in a Japanese patient with catecholaminergic polymorphic ventricular tachycardia. Case Rep Genet 8:9056596. https://doi.org/10.1155/2019/9056596
Gaburjakova M, Bal NC, Gaburjakova J, Periasamy M (2013) Functional interaction between calsequestrin and ryanodine receptor in the heart. Cell Mol Life Sci 70(16):2935–2945. https://doi.org/10.1007/s00018-012-1199-7.
Gatti G, Podini P, Meldolesi J (1997) Overexpression of calsequestrin in L6 myoblasts: formation of endoplasmic reticulum subdomains and their evolution into discrete vacuoles where aggregates of the protein are specifically accumulated. Mol Biol Cell 8(9):1789–1803
Gatti G, Trifari S, Mesaeli N, Parker JM, Michalak M, Meldolesi J (2001) Head-to-tail oligomerization of calsequestrin: a novel mechanism for heterogeneous distribution of endoplasmic reticulum luminal proteins. J Cell Biol 6(3):525–534
Gergs U, Fahrion CM, Bock P, Fisher M, Wache H, Hauptmann S, Schmits W, Neumann J (2017) Evidence for a functional role of calsequestrin 2 in mouse atrium. Acta Physiol 219:671–684
Györke S, Györke I, Terentyev D, Viatchenko-Karpinski S, Williams SC (2004a) Modulation of sarcoplasmic reticulum calcium release by calsequestrin in cardiac myocytes. Biol Res 37(4):603–607
Györke I, Hester N, Jones LR, Györke S (2004b) The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86(4):2121–2128
Glover L, Quinn S, Ryan M, Pette D, Ohlendieck K (2002) Supramolecular calsequestrin complex. Eur J Biochem 269(18):4607–4616
Glukhov AV, Kalyanasundaram A, Lou Q, Hage LT, Hansen BJ, Belevych AE, Mohler PJ, Knollmann BC, Periasamy M, Gyorke S, Fedorov VV (2015) Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex. Eur Heart J 36:686–697. https://doi.org/10.1093/eurheartj/eht452
Gray B, Bagnall RD, Lam L, Ingles J, Turner C, Haan E, Davis A, Yang PC, Clancy CE, Sy RW, Semsarian C (2016) A novel heterozygous mutation in cardiac calsequestrin causes autosomal dominant catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 13(8):1652–1660. https://doi.org/10.1016/j.hrthm.2016.05.004
Guarnier FA, Michelucci A, Serano M, Pietrangelo L, Pecorai C, Boncompagni S, Protasi F (2018) Aerobic training prevents heatstrokes in calsequestrin-1 knockout mice by reducing oxidative stress. Oxid Med Cell Longev. https://doi.org/10.1155/2018/4652480
Guo W, Campbell KP (1995) Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J Biol Chem 21(16):9027–9030
Handhle A, Ormonde CE, Thomas NL, Bralesford C, Williams AJ, Lai FA, Zissimopoulos S (2016) Calsequestrin interacts directly with the cardiac ryanodine receptor luminal domain. J Cell Sci 1(21):3983–3988
Hayakawa K, Swenson L, Baksh S, Wei Y, Michalak M, Derewenda ZS (1994) Crystallization of canine cardiac calsequestrin. J Mol Biol 7(1):357–360
Henson JH, Begg DA, Beaulieu SM, Fishkind DJ, Bonder EM, Terasaki M, Lebeche D, Kaminer B (1989) A calsequestrin-like protein in the endoplasmic reticulum of the sea urchin: localization and dynamics in the egg and first cell cycle embryo. J Cell Biol 109(1):149–161
Herzog A, Szegedi C, Jona I, Herberg FW, Varsanyi M (2000) Surface plasmon resonance studies prove the interaction of skeletal muscle sarcoplasmic reticular Ca2+ release channel/ryanodine receptor with calsequestrin. FEBS Lett 472:73–77
Houle TD, Ram ML, Cala SE (2004) Calsequestrin mutant D307H exhibits depressed binding to its protein targets and a depressed response to calcium. Cardiovasc Res 64(2):227–233
Houle TD, Ram ML, McMurray WJ, Cala SE (2006) Different endoplasmic reticulum trafficking and processing pathways for calsequestrin (CSQ) and epitope-tagged CSQ. Exp Cell Res 312(20):4150–4161
Jones LR, Zhang L, Sanborn K, Jorgensen AO, Kelley J (1995) Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem 22(51):30787–30796
Jones LR, Suzuki YJ, Wang W, Kobayashi YM, Ramesh V, Franzini-Armstrong C, Cleemann L, Morad M (1998) Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest 1(7):1385–1385
Josephs K, Patel K, Janson CM, Montagna C, McDonald TV (2017) Compound heterozygous CASQ2 mutations and long-term course of catecholaminergic polymorphic ventricular tachycardia. Mol Genet Genom Med 5(6):788–794. https://doi.org/10.1002/mgg3.323
Kraeva N, Zvaritch E, Frodis W, Sizova O, Kraev A, MacLennan DH, Riazi S. (2013) CASQ1 gene is an unlikely candidate for malignant hyperthermia susceptibility in the North American population. Anesthesiology 118:344–349
Kalyanasundaram A, Bal NC, Franzini-Armstrong C, Knollmann BC, Periasamy M (2009) The calsequestrin mutation CASQ2D307H does not affect protein stability and targeting to the junctional sarcoplasmic reticulum but compromises its dynamic regulation of calcium buffering. J Biol Chem 29(5):3076–3083. https://doi.org/10.1074/jbc.M109.053892
Kawamura M, Ohno S, Naiki N, Nagaoka I, Dochi K, Wang Q, Hasegawa K, Kimura H, Miyamoto A, Mizusawa Y, Itoh H, Makiyama T, Sumitomo N, Ushinohama H, Oyama K, Murakoshi N, Aonuma K, Horigome H, Honda T, Yoshinaga M, Ito M, Horie M (2013) Genetic background of catecholaminergic polymorphic ventricular tachycardia in Japan. Circ J 77(7):1705–1713
Kawasaki T, Kasai M (1994) Regulation of calcium channel in sarcoplasmic reticulum by calsequestrin. Biochem Biophys Res Commun 30(3):1120–1127
Kim E, Youn B, Kemper L, Campbell C, Milting H, Varsanyi M, Kang C (2007) Characterization of human cardiac calsequestrin and its deleterious mutants. J Mol Biol 2(4):1047–1057
Kirchhefer U, Wehrmeister D, Postma AV, Pohlentz G, Mormann M, Kucerova D, Müller FU, Schmitz W, Schulze-Bahr E, Wilde AA, Neumann J (2010) The human CASQ2 mutation K206N is associated with hyperglycosylation and altered cellular calcium handling. J Mol Cell Cardiol 49(1):95–105. https://doi.org/10.1016/j.yjmcc.2010.03.006
Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K (2006) Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2 + release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 116(9):2510–2520
Krause KH, Chou M, Thomas MA, Sjolund RD, Campbell KP (1989) Plant cells contain calsequestrin. J Biol Chem 15(8):4269–4272
Kobayashi YM, Alseikhan BA, Jones LR (2000) Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. J Biol Chem 275(23):17639–17646
Kumar A, Chakravarty H, Bal NC, Balaraju T, Jena N, Misra G, Bal C, Pieroni E, Periasamy M, Sharon A (2013) Identification of calcium binding sites on calsequestrin 1 and their implications for polymerization. Mol Biosyst 9(7):1949–1957. https://doi.org/10.1039/c3mb25588c
Lacruz RS, Feske S (2015) Diseases caused by mutations in ORAI1 and STIM1. Ann N Y Acad Sci 1356:45–79
Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M (2001) A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet 69(6):1378–1384
Lahat H, Pras E, Eldar M (2003) RYR2 and CASQ2 mutations in patients suffering from catecholaminergic polymorphic ventricular tachycardia. Circulation 28(3):e29
Lahat H, Pras E, Eldar M (2004) A missense mutation in CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Ann Med 36(Suppl 1):87–91
Launikonis BS, Ríos E (2007) Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol 583(1):81–97
Launikonis BS, Murphy RM, Edwards JN (2010) Toward the roles of store-operated Ca2+ entry in skeletal muscle. Pflug Arch 460(5):813–823
Lee JM, Noguchi S (2016) Calcium dyshomeostasis in tubular aggregate myopathy. Int J Mol Sci 22(11):e1952
Lewis KM, Ronish LA, Ríos E, Kang C (2015) Characterization of two human skeletal calsequestrin mutants implicated in malignant hyperthermia and vacuolar aggregate myopathy. J Biol Chem 290(48):28665–28674. https://doi.org/10.1074/jbc.M115.686261
Lunz V, Romanin C, Frischauf I (2019) STIM1 activation of Orai1. Cell Calcium 77:29–38
Ma J, Pan Z (2003) Retrograde activation of store-operated calcium channel. Cell Calcium 33(5–6):375–384
MacLennan DH, Wong PTS (1971) Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci 68(6):1231–1235
Maguire PB, Briggs FN, Lennon NJ, Ohlendieck K (1997) Oligomerization is an intrinsic property of calsequestrin in normal and transformed skeletal muscle. Biochem Biophys Res Commun 26(3):721–727
Maguire PB, Lennon NJ, Ohlendieck K (1998) Oligomerisation of calsequestrin from rabbit skeletal muscle. Biochem Soc Trans 26(3):S292
Maizels L, Huber I, Arbel G, Tijsen AT, Gepstein A, Khoury A, Gepstein L (2017) Patient-specific drug screening using a human induced pluripotent stem cell model of catecholaminergic polymorphic ventricular tachycardia type 2. Circ Arrhythm Electrophysiol 10:e004725
Manno C, Figueroa LC, Gillespie D, Fitts R, Kang C, Franzini-Armstrong C, Rios E (2017) Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc Natl Acad Sci USA 114(4):E638–E647
McFarland TP, Sleiman NH, Yaeger DB, Cala SE (2011) The cytosolic protein kinase CK2 phosphorylates cardiac calsequestrin in intact cells. Mol Cell Biochem 353(1–2):81–91. https://doi.org/10.1007/s11010-011-0777-6
Michelucci A, Paolini C, Canato M, Wei-Lapierre L, Pietrangelo L, De Marco A, Reggiani C, Dirksen RT, Protasi F (2015) Anti-oxidants protects calsequestrin-1 knockout mice from halothane- and heat- induced sudden death. Anesthesiology 123(3):603–617. https://doi.org/10.1097/ALN.0000000000000748
Michelucci A, Paolini C, Boncompagni S, Canato M, Reggiani C, Protasi F (2017a) Strenuous exercise triggers a life-threatening response in mice susceptible to Malignant Hyperthermia. FASEB J 31(8):3649–3662. https://doi.org/10.1096/fj.201601292R
Michelucci A, De Marco A, Guarnier F, Protasi F, Boncompagni S (2017b) Antioxidant treatment reduces formation of structural cores and improves muscle function in RYR1Y522S/WT mice. Oxid Med Cell Longev 2017:6792694. https://doi.org/10.1155/2017/6792694
Michelucci A, García-Castañeda M, Boncompagni S, Dirksen RT (2018) Role of STIM1/ORAI1-mediated store-operated Ca2+ entry in skeletal muscle physiology and disease. Cell Calcium 76:101–115
Michelucci A, Boncompagni S, Pietrangelo L, García-Castañeda M, Takano T, Malik S, Dirksen RT, Protasi F (2019) Transverse tubule remodeling enhances Orai1-dependent Ca2+ entry in skeletal muscle. Elife 28(8):e47576. https://doi.org/10.7554/eLife.47576
Mitchell RD, Simmerman HK, Jones LR (1988) Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem 25(3):1376–1381
Nesin V, Wiley G, Kousi M, Ong EC, Lehmann T, Nicholl DJ, Suri M, Shahrizaila N, Katsanis N, Gaffney PM, Wierenga KJ, Tsiokas L (2014) Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc Natl Acad Sci USA 111(11):4197–4202
Nori A, Gola E, Tosato S, Cantini M, Volpe P (1999) Targeting of calsequestrin to sarcoplasmic reticulum after deletions of its acidic carboxy terminus. Am J Physiol 277(5):C974–C981. https://doi.org/10.1152/ajpcell.1999.277.5.C974
Nori A, Furlan S, Patiri F, Cantini M, Volpe P (2000) Site-directed mutagenesis and deletion of three phosphorylation sites of calsequestrin of skeletal muscle sarcoplasmic reticulum. Effects on intracellular targeting. Exp Cell Res 10(1):40–49
Nori A, Valle G, Massimino ML, Volpe P (2001) Targeting of calsequestrin to the sarcoplasmic reticulum of skeletal muscle upon deletion of its glycosylation site. Exp Cell Res 15(1):104 –104
Nori A, Bortoloso E, Frasson F, Valle G, Volpe P (2004) Vesicle budding from endoplasmic reticulum is involved in calsequestrin routing to sarcoplasmic reticulum of skeletal muscles. Biochem J 15(Pt 2):505–512
Nori A, Valle G, Bortoloso E, Turcato F, Volpe P (2006) Calsequestrin targeting to sarcoplasmic reticulum of skeletal muscle fibers. Am J Physiol Cell Physiol 291(2):C245–C253
Novak A, Barad L, Zeevi-Levin N, Shick R, Shtrichman R, Lorber A, Itskovitz-Eldor J, Binah O (2012) Cardiomyocytes generated from CPVT D307H patients are arrhythmogenic in response to β-adrenergic stimulation. J Cell Mol Med 16(3):468–482. https://doi.org/10.1111/j.1582-4934.2011.01476.x
Oddoux S, Brocard J, Schweitzer A, Szentesi P, Giannesini B, Brocard J, Fauré J, Pernet-Gallay K, Bendahan D, Lunardi J, Csernoch L, Marty I (2009) Triadin deletion induces impaired skeletal muscle function. J Biol Chem 11(50):34918–34929. https://doi.org/10.1074/jbc.M109.022442
Ohkura M, Furukawa K, Fujimori H, Kuruma A, Kawano S, Hiraoka M, Kuniyasu A, Nakayama H, Ohizumi Y (1998) Dual regulation of the skeletal muscle ryanodine receptor by triadin and calsequestrin. Biochemistry 15(37):12987–12993
Okuma H, Saito F, Mitsui J, Hara Y, Hatanaka Y, Ikeda M, Shimizu T, Matsumura K, Shimizu J, Tsuji S, Sonoo M (2016) Tubular aggregate myopathy caused by a novel mutation in the cytoplasmic domain of STIM1. Neurol Genet 2(1):e50
Pan Z, Brotto M, Ma J (2014) Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep 47(2):69–79
Paolini C, Quarta M, Nori A, Boncompagni S, Canato M, Volpe P, Allen PD, Reggiani C, Protasi F (2007) Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J Physiol 583(Pt 2):767–784
Paolini C, Quarta M, D’Onofrio L, Reggiani C, Protasi F (2011) Differential effect of calsequestrin ablation on structure and function of fast and slow skeletal muscle fibers. J Biomed Biotechnol. https://doi.org/10.1155/2011/634075
Pape PC, Fénelon K, Lamboley CR, Stachura D (2007) Role of calsequestrin evaluated from changes in free and total calcium concentrations in the sarcoplasmic reticulum of frog cut skeletal muscle fibres. J Physiol 15:319–367
Park H, Wu S, Dunker AK, Kang C (2003) Polymerization of calsequestrin. Implications for Ca2+ regulation. J Biol Chem 2(18):16176–16182
Park H, Park IY, Kim E, Youn B, Fields K, Dunker AK, Kang C (2004) Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J Biol Chem 23(17):18026–18033
Perni S, Close M, Franzini-Armstrong C (2013) Novel details of calsequestrin gel conformation in situ. J Biol Chem 25(43):31358–31362. https://doi.org/10.1074/jbc.M113.507749
Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, Mannens MM, Wilde AA, Guicheney P (2002) Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res 91(8):e21–26
Protasi F, Paolini C, Dainese M (2009) Calsequestrin-1: a new candidate gene for malignant hyperthermia (MH) and environmental heat stroke (EHS). J Physiol 587:3095–3100. https://doi.org/10.1113/jphysiol.2009.171967
Protasi F, Paolini C, Canato M, Reggiani C, Quarta M (2011) Lessons from calsequestrin-1 ablation in vivo: much more than a Ca(2+) buffer after all. J Muscle Res Cell Motil 32(4–5):257–720. https://doi.org/10.1007/s10974-011-9277-2
Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, Volpe P, Fill M (2008) Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen Physiol 131(4):325-325 34. https://doi.org/10.1085/jgp.200709907
Qin J, Valle G, Nani A, Chen H, Ramos-Franco J, Nori A, Volpe P, Fill M (2009) Ryanodine receptor luminal Ca2+ regulation: swapping calsequestrin and channel isoforms. Biophys J 7(7):1961–1970. https://doi.org/10.1016/j.bpj.2009.07.030
Ram ML, Kiarash A, Marsh JD, Cala SE (2004) Phosphorylation and dephosphorylation of calsequestrin on CK2-sensitive sites in heart. Mol Cell Biochem 266(1–2):209–217
Rani S, Park CS, Sreenivasaiah PK, Kim DH (2016) Characterization of Ca(2+)-dependent protein-protein interactions within the Ca(2+) release units of cardiac sarcoplasmic reticulum. Mol Cells 39(2):149–515. https://doi.org/10.14348/molcells.2016.2284
Rossi D, Bencini C, Maritati M, Benini F, Lorenzini S, Pierantozzi E, Scarcella AM, Paolini C, Protasi F, Sorrentino V (2014a) Distinct regions of triadin are required for targeting and retention at the junctional domain of the sarcoplasmic reticulum. Biochem J 458(2):407–17. https://doi.org/10.1042/BJ20130719
Rossi D, Vezzani B, Galli L, Paolini C, Toniolo L, Pierantozzi E, Spinozzi S, Barone V, Pegoraro E, Bello L, Cenacchi G, Vattemi G, Tomelleri G, Ricci G, Siciliano G, Protasi F, Reggiani C, Sorrentino V (2014b) A mutation in the gene causes a vacuolar myopathy with accumulation of sarcoplasmic reticulum protein aggregates. Hum Mutat 35(10):1163–1170
Royer L, Ríos E (2009) Deconstructing calsequestrin. Complex buffering in the calcium store of skeletal muscle. J Physiol 587(Pt 13):3101–3111. https://doi.org/10.1113/jphysiol.2009.171934
Sacchetto R, Volpe P, Damiani E, Margreth A (1993) Postnatal development of rabbit fast-twitch skeletal muscle: accumulation, isoform transition and fibre distribution of calsequestrin. J Muscle Res Cell Motil 14(6):646–653
Sanchez EJ, Munske GR, Criswell A, Milting H, Dunker AK, Kang C (2011) Phosphorylation of human calsequestrin: implications for calcium regulation. Mol Cell Biochem 353(1–2):195–204. https://doi.org/10.1007/s11010-011-0787-4
Sanchez EJ, Lewis KM, Danna BR, Kang C (2012a) High-capacity Ca2+ binding of human skeletal calsequestrin. J Biol Chem 30(14):11592–11601. https://doi.org/10.1074/jbc.M111.335075
Sanchez EJ, Lewis KM, Munske GR, Nissen MS, Kang C (2012b) Glycosylation of skeletal calsequestrin: implications for its function. J Biol Chem 27(5):3042–3050. https://doi.org/10.1074/jbc.M111.326363
Sato Y, Ferguson DG, Sako H, Dorn GW II, Kadambi VJ, Yatani A, Hoit BD, Walsh RA, Kranias EG (1998) Cardiac-specific overexpression of mouse cardiac calsequestrin is associated with depressed cardiovascular function and hypertrophy in transgenic mice. J Biol Chem. 273(43):28470–28477
Schmidt AG, Kadambi VJ, Ball N, Sato Y, Walsh RA, Kranias EG, Hoit BD (2000) Cardiac-specific overexpression of calsequestrin results in left ventricular hypertrophy, depressed force-frequency relation and pulsus alternans in vivo. J Mol Cell Cardiol 32(9):1735–1744
Shin DW, Ma J, Kim DH (2000) The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca(2+) and interacts with triadin. FEBS Lett 8(2):178–182
Shin DW, Pan Z, Kim EK, Lee JM, Bhat MB, Parness J, Kim DH, Ma J (2003) A retrograde signal from calsequestrin for the regulation of store-operated Ca2+ entry in skeletal muscle. J Biol Chem 278(5):3286–3292
Sparsø T, Hussain MS, Borch-Johnsen K, Jørgensen T, Madsbad S, Hansen T, Pedersen O, Andersen G (2007) Studies of association of the CASQ1 rs2275703 polymorphism in relation to type 2 diabetes and related quantitative metabolic traits among 7088 Danish whites. Mol Genet Metab 92(3):278-278 82
Stiber JA, Hawkins A, Zhang Z-S, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P (2008) STIM1 signaling controls store operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol 10(6):688–697
Stiber JA, Rosenberg PB (2011) The role of store-operated calcium influx in skeletal muscle signaling. Cell Calcium 49(5):341–349
Sztretye M, Yi J, Figueroa L, Zhou J, Royer L, Allen P, Brum G, Ríos E (2011) Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca2+ release in skeletal muscle. J Gen Physiol 138(2):231–247. https://doi.org/10.1085/jgp.201010592
Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S (2006) Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res 98(9):1151–1158
Thomas K, Navarro J, Benson RJ, Campbell KP, Rotundo RL, Fine RE (1989) Newly synthesized calsequestrin, destined for the sarcoplasmic reticulum, is contained in early/intermediate Golgi-derived clathrin-coated vesicles. J Biol Chem 25(6):3140–3145
Tijskens P, Jones LR, Franzini-Armstrong C (2003) Junctin and calsequestrin overexpression in cardiac muscle: the role of junctin and the synthetic and delivery pathways for the two proteins. J Mol Cell Cardiol 35(8):961–974
Tomasi M, Canato M, Paolini C, Dainese M, Reggiani C, Volpe P, Protasi F, Nori A (2012) Calsequestrin (CASQ1) rescues function and structure of calcium release units in skeletal muscles of CASQ1-null mice. Am J Physiol Cell Physiol 1(3):C575–C586. https://doi.org/10.1152/ajpcell.00119.2011
Tomelleri G, Palmucci L, Tonin P, Mongini T, Marini M, L’erario R, Rizzuto N, Vattemi G (2006) SERCA1 and calsequestrin storage myopathy: a new surplus protein myopathy. Brain 129(8):2085–2092
Viatchenko-Karpinski S, Terentyev D, Györke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Györke S (2004) Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ Res 5(4):471–477
Volpe P, Simon BJ (1991) The bulk of Ca2+ released to the myoplasm is free in the sarcoplasmic reticulum and does not unbind from calsequestrin. FEBS Lett 28(2):274–278
Wang S, Trumble WR, Liao H, Wesson CR, Dunker AK, Kang CH (1998) Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat Struct Biol 5(6):476–476
Wang W, Cleemann L, Jones LR, Morad M (2000) Modulation of focal and global Ca2+ release in calsequestrin-overexpressing mouse cardiomyocytes. J Physiol 15(524 Pt 2):399–414
Wang Y, Xu L, Duan H, Pasek DA, Eu JP, Meissner G (2006) Knocking down type 2 but not type 1 calsequestrin reduces calcium sequestration and release in C2C12 skeletal muscle myotubes. J Biol Chem 2(22):15572–15581
Wang L, Zhang L, Li S, Zheng Y, Yan X, Chen M, Wang H, Putney JW, Luo D (2015) Retrograde regulation of STIM1-Orai1 interaction and store-operated Ca2+ entry by calsequestrin. Sci Rep 18(5):11349. https://doi.org/10.1038/srep11349
Wang Q, Groenendyk J, Paskevicius T, Qin W, Kor KC, Liu Y, Hiess F, Knollmann BC, Wayne Chen SR, Tang J, Chen X, Agellon LB, Michalak M (2019) Two pools of IRE1a in cardiac and skeletal muscle cells. FASEB J 33:8892–8904
Wei L, Varsányi M, Dulhunty AF, Beard NA (2006) The conformation of calsequestrin determines its ability to regulate skeletal ryanodine receptors. Biophys J 15(4):1288–1301
Wei L, Hanna AD, Beard NA, Dulhunty AF (2009a) Unique isoform-specific properties of calsequestrin in the heart and skeletal muscle. Cell Calcium 45(5):474–484. https://doi.org/10.1016/j.ceca.2009.03.006
Wei L, Gallant EM, Dulhunty AF, Beard NA (2009b) Junctin and triadin each activate skeletal ryanodine receptors but junctin alone mediates functional interactions with calsequestrin. Int J Biochem Cell Biol 41(11):2214–2224. https://doi.org/10.1016/j.biocel.2009.04.017
Zima AV, Bovo E, Bers DM, Blatter LA (2010) Ca²+ spark-dependent and -independent sarcoplasmic reticulum Ca²+ leak in normal and failing rabbit ventricular myocytes. J Physiol 588:4743–4757. https://doi.org/10.1113/jphysiol.2010.197913
Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR (1997) Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem 12(37):23389–23397
Zhang L, Wang L, Li S, Xue J, Luo D (2016) Calsequestrin-1 regulates store-operated Ca2+ entry by inhibiting STIM1 aggregation. Cell Physiol Biochem 8(6):2183–2193. https://doi.org/10.1159/000445574
Zhao X, Min CK, Ko JK, Parness J, Kim DH, Weisleder N, Ma J (2010) Increased store-operated Ca2+ entry in skeletal muscle with reduced calsequestrin-1 expression. Biophys J 99(5):1556–1564. https://doi.org/10.1016/j.bpj.2010.06.050
Funding
Funding was provided by Telethon Grant No. GGP19231A and PRIN 2015 Grant No. 2015ZZR4W3 to VS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare not to have conflicts of interest or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rossi, D., Gamberucci, A., Pierantozzi, E. et al. Calsequestrin, a key protein in striated muscle health and disease. J Muscle Res Cell Motil 42, 267–279 (2021). https://doi.org/10.1007/s10974-020-09583-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-020-09583-6