Abstract
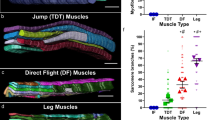
Stretchin-klp is a newly described protein in Drosophila indirect flight muscles (IFM) that migrates on SDS gels as two distinct components of approximately 225 and 231 kD. Although the larger isoform is IFM specific, the smaller stretchin-klp isoform is expressed not only in IFM, but also in wild-type tissues of the adult head, abdomen and thorax from which the IFM has been removed. It is not detected, however, in jump or leg muscles. Probes derived from a cDNA encoding part of stretchin-klp hybridize with a 6.7 kb mRNA. Stretchin-klp is one of several putative products of the Stretchin-Myosin light chain kinase gene and is predicted to have multiple immunoglobulin domains arranged in tandem pairs separated by variable length spacers. Polyclonal antibodies directed against the expressed peptide of the stretchin-klp cDNA label the IFM myofibril A-band, though not its central and lateral regions. Analyses of IFM mutants indicate that the larger stretchin-klp isoform is myosin dependent. Although the normal adult myosin filament or the ‘headless’ myosin rod is sufficient for accumulation of both the large and small stretchin-klp isoforms, loss of myosin, or substitution of the adult rod with an embryonic one in IFM prevents the larger isoform from being formed or stabilized. During development stretchin-klp is first detected at pupal stage p8, when myofibrils are being constructed. These studies suggest that this newly identified protein is a major component of the Drosophila IFM thick filament.
Similar content being viewed by others
References
Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, et al., (2000). The genome sequence of Drosophila melanogaster Science 287:2185–2195
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ, (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs Nucl Acids Res 25:3389–3402
Arbeitman MN, Furlong EEM, Imam F, Johnson E, Null BH, Baker BS, Krasnow MA, Scott MP, Davis RW, White KP, (2002). Gene expression during the life cycle of Drosophila melanogaster Science 297:2270–2275
Ashhurst DE, Cullen MJ, (1977). The structure of fibrillar flight muscle. In: Tregear RT, (ed). Insect Flight Muscle. North-Holland Publishing Company, New York, 9–14
Auerbach D, Bantle S, Keller S, Hinderling V, Leu M, Ehler E, Perriard J, (1999). Different domains of the M-Band protein myomesin are involved in myosin binding and M-Band targeting Mol Bio Cell 10:1297–1308
Ayer G, Vigoreaux JO, (2003). Flightin is a myosin rod binding protein Cell Biochem Biophys 38:41–54
Ayme-Southgate A, Bounaix C, Riebe TE, Southgate R, (2004). Assembly of the giant protein projectin during myofibrillogenesis in Drosophila indirect flight muscles BMC Cell Biol 5:17
Bainbridge SP, Bownes M, (1981). Staging the metamorphosis of Drosophila melanogaster J Embryol Exp Morphol 66:57–80
Ball E, Karlik CC, Beall CJ, Saville DL, Sparrow JC, Bullard B, Fyrberg EA, (1987). Arthrin, a myofibrillar protein of insect flight muscle, is an Actin-Ubiquitin Conjugate Cell 51:221–228
Barton A, Ayer G, Heymann N, Maughan DW, Lehmann FO, Vigoreaux JO, (2005). Flight muscle properties and aerodynamic performance of Drosophila expressing a flightin transgene J Exp Biol 208:549–560
Becker KD, O’Donnell PT, Heitz JM, Vito M, Bernstein SI, (1992). Analysis of Drosophila paramyosin: identification of a novel isoform which is restricted to a subset of adult muscles J Cell Biol 116:669–681
Bernstein SI, O’Donnell PT, Cripps RM, (1993). Molecular genetic analysis of muscle development, structure, and function in Drosophila Int Rev Cytol 143:63–152
Bullard B, Goulding D, Ferguson C, Leonard K, (2000). Links in the chain: the contribution of kettin to the elasticity of insect muscles. In: Granzier, Pollack, (eds). Elastic Filaments of the Cell Kluwer Academic/ Plenum Publishers, 207–218
Champagne MB, Edwards KA, Erickson HP, Kiehart DP, (2000). Drosophila Stretchin-MLCK is a novel member of the Titin/Myosin light chain kinase family J Mol Biol 300:759–777
Chun M, Falkenthal S, (1988). Ifm(2)2 is a myosin heavy chain allele that disrupts myofibrillar assembly only in the indirect flight muscle of Drosophila melanogaster J Cell Biol 107
Cripps RM, Suggs JA, Bernstein SI, (1999). Assembly of thick filaments and myofibrils occurs in the absence of the myosin head EMBO J 18:1793–1804
Falkenthal S, Graham M, Wilkinson J, (1987). The indirect flight muscle of Drosophila accumulates a unique myosin alkali light chain isoform Dev Biol 121:263–272
Friederich E, Vancompernolle K, Huet C, Goethals M, Finidori J, Vandekerckhove J, Louvard D, (1992). An actin-binding site containing a conserved motif of charged amino acid residues is essential for the morphogenic effect of villin Cell 70:81–92
Fujita SC, Inoue H, Yoshioka T, Hotta Y, (1987). Quantitative tissue isolation from Drosophila freeze-dried in acetone Biochem J 243:97–104
Gilbert R, Cohen JA, Pardo S, Basu A, Fischman DA, (1999). Identification of the A-band localization domain of myosin binding proteins C and H (MyBP-C, MyBP-H) in skeletal muscle J Cell Sci 112:69–79
Hao Y, Bernstein SI, Pollack GH, (2003). Alternative myosin S2 hinge regions in Drosophila IFM affect myofibrillar stiffness Biophys J 84:561a
Harlow E, Lane D, (1988). Antibodies, A Laboratory Manual Cold Spring Harbor Laboratory: Cold Spring Harbor, NY
Hastings GA, Emerson CPJ, (1991). Myosin functional domains encoded by alternative exons are expressed in specific thoracic muscle of Drosophila J Cell Biol 114:263–276
Hiromi Y, Hotta Y, (1985). Actin gene mutations in Drosophila: heat shock activation in the indirect flight muscles EMBO J 4:1681–1687
Houmeida A, Holt J, Tskhovrebova L, Trinick J, (1995). Studies of the interaction between titin and myosin J Cell Biol 131:1471–1481
Jewell BR, Ruegg C, (1966). Oscillatory contraction of insect fibrillar muscle after glycerol extraction Proc R Soc Lond B 164:428–459
Kenny PA, Liston EM, Higgins DG, (1999). Molecular evolution of immunoglobulin and fibronectin domains in titin and related muscle proteins Gene 232:11–23
Knudsen KA, (1985). Protein transferred to nitrocellulose for use as immunogens Anal Biochem 147:285–288
Kolmerer B, Clayton J, Benes V, Allen T, Ferguson C, Leonard K, Weber U, Knekt M, Ansorge W, Labeit S, Bullard B, (2000). Sequence and expression of the kettin gene in Drosophila melanogaster and Caenorhabditis elegans J Mol Biol 296:435–448
Kronert WA, Edwards KA, Roche ES, Wells L, Bernstein SI, (1991). Muscle-specific accumulation of Drosophila myosin heavy chains: a splicing mutation in an alternative exon results in an isoform substitution EMBO J 10:2479–2488
Kulke M, Neagoe C, Kolmerer B, Minajeva A, Hinssen H, Bullard B, Linke WA, (2001). Kettin, a major source of myofibrillar stiffness in Drosophila indirect flight muscle J Cell Biol 154:1045–1057
Laemmli UK, (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4 Nature 270:680–685
Lakey A, Labeit S, Gautel M, Gerguson C, Barlow DP, Leonard K, Bullard B, (1993). Kettin, a large modular protein in the Z-disc of insect muscles EMBO J 12:2863–2871
Levine JD, (1973). Properties of the nervous system controlling flight in Drosophila melanogaster J Comp Physiol 84
Levine JD, Wyman RJ, (1973). Neurophysiology of flight in wild-type and mutant Drosophila Proc Natl Acad Sci USA 70:1050–1054
Marden JH, (2005). Functional and ecological effects of isoform variation in insect flight muscle. In: JO Vigoreaux, (ed). Nature’s Versatile Engine: Insect Flight Muscle Inside and Out. Landes Biosciences, Georgetown, TX, 214–229
Maroto M, Arredondo J, Goulding D, Marco R, Bullard B, Cervera M, (1996). Drosophila paramyosin/miniparamyosin gene products show a large diversity in quantity, localization and isoform pattern: a possible role in muscle maturation and function J Cell Biol 134:81–92
Maroto M, Arredondo J, San Roman M, Marco R, Cervera M, (1995). Analysis of the paramyosin/miniparamyosin gene: miniparamyosin is an independently transcribed, distinct, paramyosin isoform, widely distributed in invertebrates J Biol Chem 270:4375–4382
Maughan DW, Vigoreaux JO, (1999). An integrated view of insect flight muscle: genes, motor molecules and motion News Physiol Sci 14:87–92
Miller BM, Bernstein SI, (2005). Myosin. In: JO Vigoreaux (ed). Nature’s Versatile Engine: Insect Flight Muscle Inside and Out. Landes Bioscience Georgetown, TX, 62–75
Mogami K, Hotta Y, (1981). Isolation of Drosophila flightless mutants which affect myofibrillar proteins of indirect flight muscle Mol Gen Genet 183:409–417
Obermann WM, van der Ven PF, Steiner F, Weber K, Furst DO, (1998). Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band Mol Bio Cell 9:829–840
Okagaki T, Weber FE, Fischman DA, Vaughan KT, Mikawa T, Reinach FC, (1993). The major myosin-binding domain of skeletal muscle MyBP-C (C protein) resides in the COOH-terminal, immunoglobulin C2 motif J Cell Biol 123:619–626
Palazzo MJ, Hamilton BA, Ding D, Martin CH, Mead DA, Mierendorf RC, Raghavan KV, Meyerowitz EM, Lipshitz HD, (1990). Phage lambda cDNA cloning vectors for subtractive hybridization, fusion protein synthesis and Cre loxP automatic plasmid subcloning Gene 88:25–36
Patel SR, Saide JD, (2001). A(225), a novel A-band protein of Drosophila indirect flight muscle Biophys J 80:71a
Patel SR, Saide JD, (2003). A(225), a Drosophila indirect flight muscle A-band protein, has a myosin dependent an a myosin independent isoform Biophys J 84:564a
Pearson WR, (1990). Rapid and sensitive sequence comparisons with FASTP and FASTA Method Enzym 183:63–98
Pringle JWS, (1972). Arthopod muscle. In: GH Bourne (ed). The Structure and Function of Muscle. Academic Press, New York, 491–541
Pringle JWS, (1978). The Croonian Lecture. Stretch activation of muscle; function and mechanism Proc R Soc Lond B 201:107–130
Qiu F, Brendel S, Cunha PM, Astola N, Song B, Furlong EE, Leonard KR, Bullard B, (2005). Myofilin, a protein in the thick filaments of insect muscle J Cell Sci 118:1527–1536
Reedy MC, Bullard B, Vigoreaux JO, (2000). Flightin is essential for thick filament assembly and sarcomere stability in Drosophila flight muscles J Cell Biol 151:1483–1499
Saide JD, (1981). Identification of a connecting filament protein in insect fibrillar flight muscle J Mol Biol 153:661–679
Saide JD, (2005). The Insect Z-Band. In: JO Vigoreaux, (ed). Nature’s Versatile Engine: Insect Flight Muscle Inside and Out. Landes Biosciences, Georgetown, TX, 150–166
Saide JD, Chin-Bow S, Hogan-Sheldon J, Busquets-Turner L, Vigoreaux JO, Valgeirsdottir K, Pardue ML, (1989). Characterization of components of Z-bands in the fibrillar flight muscle of Drosophila melanogaster J Cell Biol 109:2157–2167
Sambrook J, Fritsch EF, Maniatis T, (1989). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Soteriou A, Gamage M, Trinick J, (1993). A survey of interactions made by the giant protein titin J Cell Sci 104:119–123
Swank DM, Wells L, Kronert WA, Morrill GE, Bernstein SI, (2000). Determining structure/function relationships for sarcomeric myosin heavy chain by genetic and transgenic manipulation of Drosophila Microsc Res Tech 50:430–442
Takano-Ohmuro H, Takahashi S, Hirose G, Maruyama K, (1990). Phosphorylated and dephosphorylated myosin light chains of Drosophila fly and larva Comp Biochem Physiol B 95:179–181
Trombitas K, Jin JP, Granzier H, (1995). The mechanically active domain of titin in cardiac muscle Circ Res 77:856–861
van Straaten M, Goulding D, Kolmerer B, Labeit S, Clayton J, Leonard K, Bullard B, (1999). Association of kettin with actin in the Z-Disc of insect flight muscle J Mol Biol 285:1549–1562
Vigoreaux JO, (1994). Alterations in flightin phosphorylation in Drosophila flight muscles are associated with myofibrillar defects engendered by actin and myosin heavy-chain mutant alleles Biochem Genet 32:301–314
Vigoreaux JO, Saide JD, Valgeirsdottir K, Pardue ML, (1993). Flightin, a novel myofibrillar protein of Drosophila stretch-activated muscles J Cell Biol 121:587–598
Xie WQ, Rothblum LI, (1991). Rapid, small-scale RNA isolation from tissue culture cells Biotechniques 11:325–327
Acknowledgements
We are grateful to Bruce Hamilton for providing the λEXLOX adult Drosophila cDNA expression library. We thank Donald Gantz for advice with electron microscopy, Hector Lucero for guidance with molecular biology protocols, Marc Champagne for helpful discussions, Jeff Moore for comments on the manuscript and Sanford Bernstein, John Sparrow and Jim Vigoreaux for providing mutant stocks of Drosophila. Maria Ericsson (Harvard Medical School Core Electron Microscopy Facility) sectioned frozen preparations and provided technical assistance with immuno-gold staining. We are grateful to the Department of Physiology and Biophysics for supporting this work and to the former chairman of the Department of Physiology, the late Benjamin Kaminer, who was a generous, kind, encouraging mentor for over two decades.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, S.R., Saide, J.D. Stretchin-klp, a novel Drosophila indirect flight muscle protein, has both myosin dependent and independent isoforms. J Muscle Res Cell Motil 26, 213–224 (2005). https://doi.org/10.1007/s10974-005-9012-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-005-9012-y