Abstract
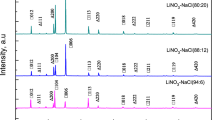
The static melting method is used to prepare KNO3–NaNO3–NaCl ternary mixed molten salt containing 5–20 mass% NaCl, and the thermal physical parameters such as specific heat capacity, melting point, and latent heat of phase change are measured by differential scanning calorimetry. The microstructures of the mixed molten salts are characterized by XRD and SEM. The experimental results show that compared with the base salt, after adding different mass fractions of NaCl, the melting point of the molten salts was reduced by about 24.8%, and the latent heat of phase change was increased by 22.71–52.53 J g−1, the solid and liquid state specific heat capacity of molten salts increased by 3.4% and 2.3% after the addition of 10 mass% NaCl. The addition of 15 mass% NaCl formed a special chlorine salt layer on the surface of the molten salt. This structure reduced the intermolecular voids and enhanced the intermolecular force, which may be the reason for the increase in specific heat capacity and latent heat of phase change.




Similar content being viewed by others
References
Hu Y, He Y, Zhang Z, Wen D. Effect of Al2O3 nanoparticle dispersion on the specific heat capacity of a eutectic binary nitrate salt for solar power applications. Energy Convers Manag. 2017;142:366–73.
Kenisarin MM. High-temperature phase change materials for thermal energy storage. Renew Sustain Energy Rev. 2010;14(3):955–70.
Nie B, Palacios A, Zou B, Liu J, Zhang T, Li Y. Review on phase change materials for cold thermal energy storage applications. Renew Sustain Energy Rev. 2020;134:110340.
Ren N, Wu YT, Ma CF, Sang LX. Preparation and thermal properties of quaternary mixed nitrate with low melting point. Sol Energy Mater Sol Cells. 2014;127:6–13.
Wanyu H, Quanying Y. Molten salt as a phase change material. Materials reports 2015;29(S1):128–130+140. (in Chinese)
Glatzmaier G. Summary report for concentrating solar power thermal storage workshop: new concepts and materials for thermal energy storage and heat-transfer fluids, May 20, 2011: National Renewable Energy Lab (NREL), Golden, CO (United States) 2011.
Liu M, Tay NS, Bell S, et al. Review on concentrating solar power plants and new developments in high temperature thermal energy storage technologies. Renew Sustain Energy Rev. 2016;53:1411–32.
Zhang Y, Li J, Gao L, Wang M. Nitrate based nanocomposite thermal storage materials: Understanding the enhancement of thermophysical properties in thermal energy storage. Sol Energy Mater Sol Cells. 2020;216:110727.
Chen X, Wu YT, Wang X, Ma CF. Experimental study on the specific heat and stability of molten salt nanofluids prepared by high-temperature melting. Sol Energy Mater Sol Cells. 2018;176:42–8.
Durth M, Prieto C, Rodríguez-Sánchez A, Patiño-Rodríguez D, Cabeza LF. Effects of sodium nitrate concentration on thermophysical properties of solar salts and on the thermal energy storage cost. Sol Energy. 2019;182:57–63.
Akilu S, Baheta A, Sharma K, Said M, editors. Experimental determination of nanofluid specific heat with SiO2 nanoparticles in different base fluids. AIP Conference Proceedings; 2017: AIP Publishing LLC.
Nithiyanantham U, González-Fernández L, Grosu Y, Zaki A, Igartua JM, Faik A. Shape effect of Al2O3 nanoparticles on the thermophysical properties and viscosity of molten salt nanofluids for TES application at CSP plants. Appl Therm Eng. 2020;169:114942.
Li Y, Yue G, Yu Y, Zhu Q. Preparation and thermal characterization of LiNO3–NaNO3–KCl ternary mixture and LiNO3–NaNO3–KCl/EG composites. Energy. 2020;196:117067.
Gasanaliev AM, Gamataeva BY. Heat-accumulating properties of melts. Russ Chem Rev. 2000;69(2):179.
He MZ, Yang L, Zhang Z. Supercooling characteristics of inorganic phase change material CaCl2·6H2O. J Chem Ind Eng. 2017;68(11):4016–24.
Arconada N, Arribas L, Lucio B, González-Aguilar J, Romero M. Macroencapsulation of sodium chloride as phase change materials for thermal energy storage. Sol Energy. 2018;167:1–9.
Zhong L, Zhang X, Luan Y, Wang G, Feng Y, Feng D. Preparation and thermal properties of porous heterogeneous composite phase change materials based on molten salts/expanded graphite. Sol Energy. 2014;107:63–73.
Li Y, Wang C, Liu G, Zhu Q, Qiu Z. Thermal property characterization of a low supercooling degree binary mixed molten salt for thermal energy storage system. Int J Thermophys. 2019;40(4):1–12.
Tian H, Wang W, Ding J, Wei X. Thermal performance and economic evaluation of NaCl–CaCl2 eutectic salt for high-temperature thermal energy storage. Energy. 2021;227:120412.
Galazutdinova Y, Vega M, Grágeda M, Cabeza LF, Ushak S. Preparation and characterization of an inorganic magnesium chloride/nitrate/graphite composite for low temperature energy storage. Sol Energy Mater Sol Cells. 2018;175:60–70.
D’Aguanno B, Karthik M, Grace A, Floris A. Thermostatic properties of nitrate molten salts and their solar and eutectic mixtures. Sci Rep. 2018;8(1):1–15.
Roget F, Favotto C, Rogez J. Study of the KNO3–LiNO3 and KNO3–NaNO3–LiNO3 eutectics as phase change materials for thermal storage in a low-temperature solar power plant. Sol Energy. 2013;95:155–69.
Acknowledgements
This research is supported by the Science and Technology Commission of Shanghai Municipality under the contract No. 20dz1205208, which is gratefully acknowledged by the authors.
Author information
Authors and Affiliations
Contributions
Y. Li prepared the samples and the manuscript. W.W. Tan and C.G. Wang contributed to the analysis and manuscript preparation. Q.Z. Zhu helped with the analysis through constructive discussions.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that there are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Authors: Y. Li, W.W. Tan, C.G. Wang, Q.Z. Zhu.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Tan, W.W., Wang, C.G. et al. Research on the effect of adding NaCl on the performance of KNO3–NaNO3 binary molten salt. J Therm Anal Calorim 148, 733–739 (2023). https://doi.org/10.1007/s10973-022-11791-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11791-w