Abstract
Polyneuropathy is defined as a simultaneous malfunction of several peripheral nerves, which could be a side effect of cancer therapy as well. Many kinds of drugs, supposedly cyclophosphamide, also can induce a disease classified as toxic polyneuropathy. It is well known that a severe problem in the locomotor activity can join to it. Recently, we have no enough information about the attacked points in the structure of muscle proteins, as well as about the change in the interaction of myosin actin. In the present study, we analyse this side effect on skeletal muscle (m. gastrocnemius) by differential scanning calorimetry (DSC), as an established thermal analysis method, to follow the possible consequence of drug treatment in the most important muscle protein. We used cyclophosphamide-treated in vitro animal model (guinea pig) with a comparable dosage and time handling of human protocol to show evidences of this drug-induced effects. According to our results, we could show a dose-dependent difference between thermal parameters (denaturation temperature and calorimetric enthalpy) of untreated and treated samples assigned to their contractile proteins (actin and myosin), which can be detected by DSC. It proved that we can create new possibilities in the detection and prognosis of expected and unwanted side effects of cyclophosphamide, such as change of locomotor activity joined to polyneuropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the twenty-first century, malignancies are the second leading cause of death after cardiovascular disease in developed countries and show a slight upward trend. During chemotherapy, cyclophosphamide is an effective immunosuppressive drug with wide application field, e.g. haematologic malignancies and in each type of solid tumours, etc. [1]. It is the reason that appeared on the most important drugs’ list published by WHO in April, 2015 [2]. It is a good tool during polyneuropathy treatment, but in several cases, it can induct this disorder too [1].
Cyclophosphamide has a wide variety of attacked points, and depending on the affected area, sensory, motor or vegetative signs of disfunction could occur, which makes more difficult to find the correct diagnosis. Locomotive human activity is controlled by the central nervous system, and the “commands” are conducted through the nerves to the muscles. The good muscle function this way depends on the actual structure/stage of nervous and muscle system, mainly on the conformation of myosin and actin and on their interaction as well as on the communication between them. Any damage or unexpected changes in protein structure caused by chemotherapeutic agents in any step will cause a malfunction. It was shown in animal model that the cyclophosphamide can cause dose-dependent effect in the thermal characteristic of nerve, different muscles as well as in different blood compounds (plasma, red blood cell) [1, 3,4,5,6,7].
The aim of this paper is to look for the possible attack point of cyclophosphamide causing muscle function damage in dose-dependent manner, which may cause serious consequences on the patient’s life and ability.
Materials and method
Animal samples
Adult guinea pigs (Cavia porcellus) were injected intraperitoneally with cyclophosphamide according to 11 different dosing schemes, administrating 1-6 times consecutively including a couple of day break (n = 55, n = 5/group, as in case [1]). The dose of cyclophosphamide was calculated according to the guinea pigs’ mass using the dosage of human protocols (150 mg/kg b.m.). Untreated guinea pigs were used as controls (n = 5).
Guinea pigs were euthanased in chambers filled with narcotic ether, and then, gastrocnemius muscles were prepared from the lower extremities. 0.5 × 0.5 × 2 cm sized muscle was removed from the animals from both side of the body. The samples were kept in sterile saline solution at 4 °C until the examinations by DSC. All animal experiments were approved by the local animal care and use committee and conformed to the current guidelines for the care and use of laboratory animals (approval number: BA02/2000-4/2012/).
DSC measurements
The prepared samples were washed three times in normal saline to remove dirty and residual tissue within 6 h before the calorimetric examinations. Samples were stored in a sterile isotonic saline on 4 °C. The analysis was made by a SETARAM Micro DSC II calorimeter between 0 and 100 °C with a heating rate of 0.3 K. Conventional Hastelloy batch vessels (V = 1 mL) were used for the experiment to determine denaturation with 950 µL sample volume (sample + buffer) in average. Samples’ masses were between 250 and 400 mg. Normal saline was used as a reference. With the help of a two-point setting, SETARAM peak integration calorimetric enthalpy was calculated from the area under the heat absorption curve and then, the results (denaturation or melting temperature (Tm) and calorimetry enthalpy (Hcal) data of samples) were compared.
Results
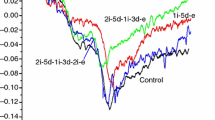
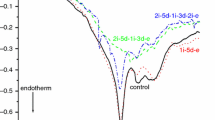
We plotted the most characteristic denaturation curves of treated m. gastrocnemius muscles in Fig. 1. At the first sight, we can see at least three well-separable thermal structural domains around 50, 58 and 65 °C. During a closer examination, two overlapping denaturation transitions can be assumed in the temperature range of 60–72 °C, and therefore, deconvolution of the averaged experimental curve was performed. Gaussian curves were used for deconvolution.
The denaturation temperatures of the supposed contributors were chosen based on our previous measurements on rabbit psoas muscles, so that the total area of the deconvoluted curves is as close as possible to the accuracy of the calorimetric enthalpy determination (equipment limit, ~ 5%). The deconvolution of averaged experimental curves in Fig. 1 can be seen in Fig. 2.
We have looked for the relative abundance of each supposed thermal transition in the total calorimetric enthalpy (see Table 1) adapting the idea of Briere et al. [8]. On the basis of these results, it can be seen quite well that the most affected muscle compounds are the myosin tail (Tm2) and F-actin (Tm3 and Tm4).
Discussion
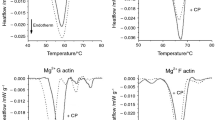
It is very important to know the thermal characteristic of basic contractile muscle proteins (actin and myosin) in case of the interpretation of the denaturation of a whole muscle. The basic unfolding characteristics of these proteins in solution were clarified at early of 1980s and finished at 1990 [9,10,11,12,13,14,15,16]. The G-actin (globular or monomer actin) can be decomposed into least two, while the F-actin into three thermal domains (as the consequence of stabilising effect of polymerisation). This was supported by determination of atomic structure of actin [17] and by combination the DSC study with EPR [18, 19]. The actin has a monomer form too (G-actin), but in the muscle cell, it is in filament (polymerised or F-actin) form. In the case of rabbit psoas G-actin, we could separate two melting peaks around 56.8 °C (subdomains 2 and 4) and 60.3 °C (subdomains 1 and 3). F-actin exhibited three decomposed transitions (63.8, 67.8 and 70.5 °C) with larger total calorimetric enthalpy [18, 19]. Myosin solution exhibited melting peaks at 44.3, 51.1 and 59.9 °C [16]. In the case of contraction model in protein solution (actomyosin solution), these peaks shifted because of producing a complex with each other to 47.2, 51.5 and 59.4 °C for myosin and 68.1 and 72.8 °C for actin.
It was very soon realised that in the different intermediate states of ATP hydrolysis cycle (ATP is the energy source of muscle contraction. Myosin, as an ATPase enzyme digesting it, transforms chemical energy into mechanical one through binding to F-actin. ATP plays also a crucial rule in the polymerisation of actin), the phosphate analogues such as aluminium and beryllium fluoride as well as vanadate play important role [20,21,22,23,24,25,26]. These analogues can shift the actin melting peak, binding into its narrow cleft, close to 80 °C. We could prove their similar effect in myofibrils, which are a real muscle model [27, 28]. We have performed our measurements on muscle fibres too, and achieved similar temperature shift for all intermediate states of ATP hydrolysis in the higher temperature range [29,30,31,32,33,34,35,36,37,38,39].
Effect of toxins (jasplakinolide and phalloidin) gives us another possibility to explain the effect of cyclophosphamide on muscle. We have observed a significant melting temperature shift into higher range in the function of toxin type and concentration [40]. we were able to estimate the extension of cooperativity (in case of phalloidin three, while in the present of jasplaklinolide seven actin monomers exhibited alteration in their thermal stability) [41].
Looking at Table 1, we can see similar cooperativity effect caused by the bound cyclophosphamide on actin monomers. The lower thermal denaturation temperatures can refer to actin monomers which have no bound cyclophosphamide in their cleft (Tm3), while those which contain it and are influenced by the cooperativity effect could be characterised by Tm4. Their slight increase in the contribution to the total calorimetric enthalpy as well as the change of total ΔHcal itself can be another sign of the concentration dependence effect of the drug. On the basis of the recent study (see Table 1), the myosin heads are less influenced, that is, the ATPase activity of myosin practically remain unchanged, while the thermal stability of myosin tail decreases (smaller melting temperature and less enthalpy contribution). The appearance of two actin populations similar to the well-known effect of nucleotides and toxins is the sign of the extent of the effect caused by cyclophosphamide.
Conclusions
Application of TA methods in the investigation of biophysical/medical problems can prove that the informations served by them can exhibit useful information about the structural change going on in different biological systems after different treatments or medical interventions. We are getting closer to use the different TA methods to give direct diagnostic information. Our result proved that with the aid of this technique, we can create new possibilities in the detection and prognosis of expected and unwanted side effects of cyclophosphamide, such as change of locomotor activity joined to polyneuropathy. Its application can prove that early stages of different diseases or some unexpected consequence of a medical treatment can be determined this way to decrease the expenses of health system and to give possibility early recognise the severe health problems.
References
Farkas P, Könczöl F, Lőrinczy D. Examination of the peripheral nerve and muscle damage in cyclophosphamide monotherapy with DSC in animal models. J Therm Anal Calorim. 2016;126:47–53.
WHO Model List of Essential Medicines (2015) http://www.who.int/selection_medicines/committees/expert/20/EML_2015_FINAL_amended_JUN2015.pdf?ua=1.
Könczöl F, Wiegand N, Nőt LG, Lőrinczy D. Examination of the cyclophosohamide-induced polyneuropathy on guinea pig sciatic nerve and gastrocnemius muscle with differential scanning calorimetry. J Therm Anal Calorim. 2014;115:2239–43.
Farkas P, Könczöl F, Lőrinczy D. Examination of the left ventricle damage in cyclophosphamide monotherapy with DSC in animal models. J Therm Anal Calorim. 2017;127:1181–5.
Farkas P, Könczöl F, Lőrinczy D. Examination of the blood plasma and red blood cells in cyclophosphamide monotherapy with DSC in animal models. J Therm Anal Calorim. 2017;127:1239–43.
Farkas PL, Lőrinczy D. New possibilities of application of differential scanning calorimetry—new clinical diagnostic methods on the horizon? Temperature. 2017;4(2):120–2.
Farkas P, Könczöl F, Lőrinczy D. New possibilities of application of DSC as a new clinical diagnostic method. J Therm Anal Calorim. 2018;133:579–89.
Briere L-AK, Brandt JM, Medley JB. Measurement of protein denaturation in human synovial fluid and its analogues using differential scanning calorimetry. J Therm Anal Calorim. 2010;102:99–106.
Samejima K, Ishioroshi M, Yashui T. Scanning calorimetric studies on thermal denaturation of myosin and its subfragment. Agric Biol Chem. 1983;47:2373–80.
Tatunashvili LV, Privalov PL. Calorimetric investigation of G-actin denaturation. Biofizika. 1984;29:583–5.
Bertazzon A, Tian GH, Tsong TY. Differential scanning calorimetric (DSC) study of thermal unfolding of myosin and its subfragments in several forms of assemblies. Biophys J. 1988;53:236.a.
Bertazzon A, Tian GH, Lamblin A, Tsong TY. Enthalpic and entropic contributions to actin stability: calorimetry, circular dichroism, and fluorescence study and effects of calcium. Biochemistry. 1990;29:291–8.
Bertazzon A, Tsong TY. Study of effects of pH on the stability of domains in myosin rod by high-resolution differential scanning calorimetry. Biochemistry. 1990;29:6453–9.
Levitsky DI. Study of thermal denaturation of the rod part of myosin molecule by microcalorimetry and intrinsic fluorescence methods. Biofizika. 1990;35:415–20.
Lőrinczi D, Hoffmann U, Pótó L, Belágyi J, Laggner P. Conformational changes in bovine heart myosin as studied by EPR and DSC techniques. Gen Physiol Biophys. 1990;9:589–603.
Shriver JW, Kamath U. Differential scanning calorimetry of the unfolding of myosin subfragment-1, subfragment-2, and heavy meromyosin. Biochemistry. 1990;29:2556–64.
Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–9.
Lőrinczy D, Belagyi J. Scanning calorimetric and EPR studies on the thermal stability of actin. Thermochim Acta. 1995;259:153–64.
Lőrinczy D, Könczöl F, Gaszner B, Belagyi J. Structural stability of actin as studied by DSC and EPR. Thermochim Acta. 1998;322:95–100.
Combeau C, Carlier M-F. Probing the mechanism of ATP hydrolysis on F-actin using vanadate and the structural analogs of phosphate BeF3− and AlF4−. J Biol Chem. 1988;263:17429–36.
Phan BC, Reisler E. Inhibition of myosin ATPase by berillium fluoride. Biochemistry. 1992;31:4787–93.
Werber MM, Peyser YM, Muhlrad A. Characterization of stable beryllium fluoride, aluminum fluoride, and vanadate containing myosin subfragment 1-nucleotide complexes. Biochemistry. 1992;31:7190–7.
Phan BC, Faller LD, Reisler E. Kinetic and equilibrium analysis of the interactions of actomyosin subfragment-1·ADP with beryllium fluoride. Biochemistry. 1993;32:7712–9.
Bobkov A, Khvorov NV, Golitsina NL, Levitsky DI. Calorimetric characterization of the stable complex of myosin subfragment 1 with ADP and beryllium fluoride. FEBS Lett. 1993;332:64–6.
Bobkov A, Levitsky DI. Differential scanning calorimetric study of the complexes of myosin subfragment 1 with nucleoside diphosphates and vanadate or beryllium fluoride. Biochemistry. 1995;34(30):9708–13.
Bombardier H, Wong P, Gicquaud C. Effects of nucleotides on the denaturation of F-actin: a differential scanning calorimetry and FTIR spectroscopy study. Biochem Biophys Res Commun. 1997;236:798–803.
Lőrinczy D, Belágyi J. Effects of nucleotide on skeletal muscle myosin unfolding in myofibrils by DSC. Biochem Biophys Res Commun. 1995;217:592–8.
Belágyi J, Lőrinczy D. Internal motions in myosin head: effect of ADP and ATP. Biochem Biophys Res Commun. 1996;219:936–40.
Lőrinczy D, Könczöl F, Farkas L, Belágyi J, Schick C. Nucleotides induced changes in muscle fibres studied by DSC and TMDSC. Thermochim Acta. 2001;377:205–10.
Lőrinczy D, Hartvig N, Belágyi J. Nucleotide analogue induces global and local changes in muscle fibres. J Therm Anal Calorim. 2001;64:651–8.
Lőrinczy D, Hartvig N, Farkas N, Belágyi J. Binding of nucleotides at the active site modulates the local and global conformation of myosin in muscle fibres. J Therm Anal Calorim. 2001;65:351–8.
Lőrinczy D, Belágyi J. Nucleotide binding induces global and local structural changes of myosin head in muscle fibres. Eur J Biochem. 2001;268:5970–6.
Lőrinczy D, Hartvig N, Belagyi J. Analysis of nucleotide myosin complexes in skeletal muscle fibres by DSC and EPR. J Biochem Biophys Method. 2002;53:75–87.
Kiss M, Belagyi J, Lőrinczy D. Vanadate (Vi) and ADP induced domain motions in myosin head by DSC and EPR. J Therm Anal Calorim. 2003;72:565–72.
Lőrinczy D, Kiss M, Belagyi J. DSC and EPR study on AMP.PNP, BeFx and AlF4 containing nucleotide complexes. J Therm Anal Calorim. 2003;72:573–80.
Lőrinczy D. Effect of nucleotides and environmental factors on the intermediate states of ATP hydrolysis cycle in skeletal muscle fibres. In: Lőrinczy D, editor. The nature of biological systems as revealed by thermal analysis. Dordrecht: Kluwer Academic Publisher; 2004. p. 159–86.
Dergez T, Könczöl F, Farkas N, Belagyi J, Lőrinczy D. DSC study of glycerol-extracted muscle fibers in intermediate states of ATP hydrolysis. J Therm Anal Calorim. 2005;80:445–9.
Lőrinczy D, Belagyi J. Intermediate states of myosin head during ATP hydrolysis cycle in psoas muscle fibres by EPR and DSC (A review). J Therm Anal Calorim. 2007;90:611–21.
Dergez T, Lőrinczy D, Könczöl F, Farkas N, Belagyi J. Differential scanning calorimetry study of glycerinated rabbit psoas muscle fibres in intermediate state of ATP hydrolysis. BMC Struct Biol. 2007;7:41–50.
Visegrády B, Lőrinczy D, Hild G, Somogyi B, Nyitrai M. The effect of phalloidin and jasplakinolide on the flexibility and thermal stability of actin filaments. FEBS Lett. 2004;565:163–6.
Visegrády B, Lőrinczy D, Hild G, Somogyi B, Nyitrai M. A simple model for the cooperative stabilisation of actin filaments by phalloidin and jasplakinolide. FEBS Lett. 2005;579:6–10.
Acknowledgements
Open access funding provided by University of Pécs (PTE). The SETARAM Micro DSC II (Caluire, France) calorimeter was supported by the grant from the Hungarian Scientific Research Found (NKFIH) CO-272.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lőrinczy, D. Cyclophosphamide treatment evoked side effects on skeletal muscle monitored by DSC. J Therm Anal Calorim 142, 1897–1901 (2020). https://doi.org/10.1007/s10973-020-09388-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09388-2