Abstract
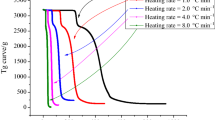
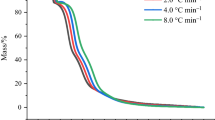
Azo compounds (azos) are widely used as radical initiators in the polymerization industry. Nonetheless, due to the azo group molecular structure, azos gravitate toward thermal decomposition and lead to thermal runaway accidents. In this paper, the thermal decomposition behaviors of 2-(1-cyano-1-methylethyl)azocarboxamide (CABN) under the dynamic and adiabatic environments were investigated using differential scanning calorimetry and accelerating rate calorimeter. Several safety assessment parameters such as time to maximum rate under adiabatic condition (TMRad), temperature of no return, and self-accelerating decomposition (SADT) temperature were calculated based on thermokinetic analysis as well as curve fitting. The results indicated that CABN decomposes at low temperatures (90.0–100.0 °C) and releases huge volumes of gaseous products, which may set off a fire, deflagration, or even explosion if the decomposition occurs uncontrolled in a confined space. Compared with commonly used azos, the shorter TMRad, lower SADT, and more heat from thermal decomposition reflect the potential thermal explosion hazards of CABN. To investigate emergency response procedure in terms of industrial applications, the oxygen-balance method was further used to evaluate the explosion hazard of CABN, and several recommendations on alleviating the thermal hazards of CABN were established to prevent catastrophic accidents.









Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor (min−1)
- C p :
-
Heat capacity of the reactant (kJ kg−1 K−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- k :
-
Rate constant (mol1−n Ln−1 s−1, n is reaction order)
- m :
-
Mass of CABN in different packaging specifications (kg)
- m T :
-
Self-heating rate at arbitrary temperature (°C min−1)
- M :
-
Molecular weight (g mol−1)
- n :
-
Reaction order
- OB:
-
Oxygen-balance
- P max :
-
Maximum pressure (bar)
- R :
-
Universal gas constant (J mol−1 K−1)
- S :
-
Wetted surface area (m2)
- SADT :
-
Self-accelerating decomposition temperature (°C)
- T :
-
Temperature at arbitrary time (K)
- T 0 :
-
Measured initial exothermic temperature (°C)
- T D24 :
-
The temperatures at which the TMRad is 24 h (°C)
- T D8 :
-
The temperatures at which the TMRad is 8 h (°C)
- T f :
-
Measured final exothermic temperature (°C)
- T m :
-
Temperature at maximum rate (K)
- T max :
-
Maximum temperature (°C)
- TMR ad :
-
Time to maximum rate under adiabatic conditions (min)
- T NR :
-
Temperature of no return (K)
- T onset :
-
Onset temperature (°C)
- T p :
-
Peak temperature (K)
- U :
-
Heat transfer coefficient (kJ min−1 m−2 K−1)
- X :
-
Number of atoms of carbon
- Y :
-
Number of atoms of hydrogen
- Z :
-
Number of atoms of oxygen
- β :
-
Heating rate (°C min−1)
- ΔH d :
-
Heat of decomposition (J g−1)
- ΔT ad :
-
Measured adiabatic temperature rise (°C)
- τ :
-
Time constant (min)
References
Idage BB, Vernekar SP, Ghatge ND. Decomposition rate studies of azo initiators in solution. J Polym Sci Polym Chem Ed. 1983;21:2145–55.
Yang Y, Tsai YT. Evaluation on thermal stability and kinetics of 2,2′-azobis(2,4-dimethyl)valeronitrile in aerobic and anaerobic conditions under isothermal process. J Therm Anal Calorim. 2018;132:1961–8.
Chiang CL, Liu SH, Cao CR, Hou HY, Shu CM. Multiapproach thermodynamic and kinetic characterization of the thermal hazards of 2,2′-azobis(2-methylpropionate) alone and when mixed with several solvents. J Loss Prev Process Ind. 2018;51:150–8.
Liu SH, Lin WC, Hou HY, Shu CM. Comprehensive runaway kinetic analysis and validation of three azo compounds using calorimetric approach and simulation. J Loss Prev Process Ind. 2017;49:970–82.
Zhang CX, Bin LuG, Chen LP, Chen WH, Peng MJ, Lv JY. Two decoupling methods for non-isothermal DSC results of AIBN decomposition. J Hazard Mater. 2015;285:61–8.
Liu SH, Cao CR, Lin WC, Shu CM. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J Hazard Mater. 2019;365:164–77.
Zhu J, Jin S, Yu Y, Zhang C, Li L, Chen S, et al. Evaluation of thermal hazards and thermo-kinetic parameters of N,N′-dinitro-4,4′-azo-bis(1,2,4-triazolone) (DNZTO). Thermochim Acta. 2016;623:58–64.
Nakamura Y, Kitada Y, Kobayashi Y, Ray B, Yamago S. Quantitative analysis of the effect of azo initiators on the structure of α-polymer chain ends in degenerative chain-transfer-mediated living radical polymerization reactions. Macromolecules. 2011;44:8388–97.
Mathias LJ, Lee S, Wright JR, Warren SC. Improvement of wood properties by impregnation with multifunctional monomers. J Appl Polym Sci. 1991;42:55–67.
Vernières-Hassimi L, Dakkoune A, Abdelouahed L, Estel L, Leveneur S. Zero-order versus intrinsic kinetics for the determination of the time to maximum rate under adiabatic conditions (TMRad): application to the decomposition of hydrogen peroxide. Ind Eng Chem Res. 2017;56:13040–9.
United Nations. Committee of experts on the transport of dangerous goods. Recommendations on the transport of dangerous goods. UN. 1995.
Halim NA, Ali A, Abidin ZHZ, Ahmad AB, Sulaiman AZ, Ibrahim ZA. Thermal analysis of organically modified Ca2+-montmorillonite using DSC and TSC techniques. J Therm Anal Calorim. 2017;128:135–40.
Alarcon RT, Gaglieri C, de Oliveira AR, Bannach G. Use of DSC in degree of conversion of dimethacrylate polymers: easier and faster than MIR technique. J Therm Anal Calorim. 2018;132:1423–7.
Ferencz A, Lőrinczy D. DSC measurements of blood plasma on patients with chronic pancreatitis and operable and inoperable pancreatic adenocarcinoma. J Therm Anal Calorim. 2017;127:1187–92.
Pakkirisamy SV, Mahadevan S, Paramashivan SS, Mandal AB. Adiabatic thermokinetics and process safety of pyrotechnic mixtures: atom bomb, Chinese, and palm leaf crackers. J Therm Anal Calorim. 2012;109:1387–95.
Lei B, Zhao W, Ziebert C, Uhlmann N, Rohde M, Seifert H. Experimental analysis of thermal runaway in 18650 cylindrical Li-ion cells using an accelerating rate calorimeter. Batteries. 2017;3:14.
Li R, Guo X, Zhang J, Zhang H, Wu C. Investigation on the thermal behavior of nitroguanidine, potassium perchlorate, ammonium perchlorate and their mixtures by DSC and TG, DEStech Transactions on Computer Science and Engineering. 2018, p. 298–307. http://www.dpi-proceedings.com/index.php/dtcse/article/view/291144
Trache D, Maggi F, Palmucci I, DeLuca LT. Thermal behavior and decomposition kinetics of composite solid propellants in the presence of amide burning rate suppressants. J Therm Anal Calorim. 2018;132:1601–15.
Doyle CD. Series approximations to the equation of thermodynamic data. Nature. 1965;207:290–1.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Fisher HG, Goetz DD. Determination of self-accelerating decomposition temperatures using the accelerating rate calorimeter. J Loss Prev Process Ind. 1991;4:305–16.
Yang D, Koseki H, Hasegawa K. Predicting the self-accelerating decomposition temperature (SADT) of organic peroxides based on non-isothermal decomposition behavior. J Loss Prev Process Ind. 2003;16:411–6.
Sun JH, Lu SX, Sun ZH. Study on thermal risk evaluation of reactive substance. China Saf Sci J. 2003;2003:44–7.
Shanley ES, Melhem GA. A review of ASTM CHETAH 7.0 hazard evaluation criteria. J Loss Prev Process Ind. 1995;8:261–4.
Shanley ES, Melhem GA. The oxygen balance criterion for thermal hazards assessment. Process Saf Prog. 1995;14:29–31.
Liu SH, Shu CM. Advanced technology of thermal decomposition for AMBN and ABVN by DSC and VSP2. J Therm Anal Calorim. 2015;121:533–40.
Guo S, Wan W, Chen C, Chen WH. Thermal decomposition kinetic evaluation and its thermal hazards prediction of AIBN. J Therm Anal Calorim. 2013;113:1169–76.
Cisneros LO, Rogers WJ, Mannan MS. Effect of air in the thermal decomposition of 50 mass% hydroxylamine/water. J Hazard Mater. 2002;95:13–25.
Wahl RUR, Zeng L, Madison SA, DePinto RL, Shay BJ. Mechanistic studies on the decomposition of water soluble azo-radical-initiators. J Chem Soc Perkin Trans. 1998;2:2009–18.
Hammond GS, Chin-Hua SW, Trapp OD, Warkentin J, Keys RT. The mechanism of decomposition of azo compounds. II. Cage effects in the decomposition of α,α′-azoisobutyronitrile and related compounds. J Am Chem Soc. 1960;82:5394–9.
Bickel AF, Waters WA. The decomposition of aliphatic azo-compounds. I. Recl des Trav Chim des Pays-Bas. 1950;69:312–20.
Eliason MA, Hirschfelder JO. General collision theory treatment for the rate of bimolecular, gas phase reactions. J Chem Phys. 1959;30:1426–36.
Roduit B, Hartmann M, Folly P, Sarbach A, Brodard P, Baltensperger R. Determination of thermal hazard from DSC measurements. Investigation of self-accelerating decomposition temperature (SADT) of AIBN. J Therm Anal Calorim. 2014;117:1017–26.
Malow M, Wehrstedt KD, Manolov M. Thermal decomposition of AIBN part A: decomposition in real scale packages and SADT determination. Thermochim Acta. 2015;621:1–5.
Cao W, Guo J, Chen X, Ding Z, Xu K, Song J, et al. Synthesis, characterization, thermal properties and theoretical investigation on Bis (guanidinium) 4,4′-azo-1h-1, 2,4-triazol-5-one. J Mol Struct. 2017;1147:754–62.
Yang LF, Sheng JJ. Feasibility of using the Frank-Kamenetskii theory to predict the spontaneous ignition of crude oil-sand mixture. Energy Fuels. 2019;33:4816–25.
Acknowledgements
The authors would like to express their appreciation to the Anhui Provincial Natural Science Foundation, China, for its financial support of this study under the Contract No. 1908085ME125.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, YM., Liu, SH. & Shu, CM. Evaluation of thermal hazards based on thermokinetic parameters of 2-(1-cyano-1-methylethyl)azocarboxamide by ARC and DSC. J Therm Anal Calorim 138, 2873–2881 (2019). https://doi.org/10.1007/s10973-019-08827-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08827-z