Abstract
The curing behaviour of the composition of a liquid-crystalline diepoxy monomer (LCEM) with the central triaromatic mesogenic group was studied using differential scanning calorimetry, conventional DSC and temperature-modulated TMDSC and nuclear magnetic resonance 1H-NMR spectroscopy. The stoichiometric amount of primary aromatic diamine, 4,4′-diaminodiphenylmethane (DDM), was used as a curing agent. TMDSC TOPEM® allowed the separation of the thermal effects related to reversible processes (e.g. phase transitions) from irreversible processes (cross-linking reaction) and determining that irreversible curing reaction occurs at the same time as reversible transition of the LCEM from crystal to the liquid-crystalline nematic phase. Additionally, 1H-NMR analysis of the LCEM/DDM mixture preheated to a temperature from the temperature range of complex thermal changes was conducted. The obtained spectrum clearly showed the presence of partially cured products in the investigated samples and additionally proved that during the endothermic phase transition of the LCEM monomer an exothermic cross-linking reaction was occurring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liquid-crystalline (LC) compounds have been known for over 100 years, since they were discovered by Friedrich Reinitzer at the end of the 19th century [1]. Besides the low molecular mass liquid crystals, the solid polymeric liquid-crystalline materials are synthesized or analysed, including non-cross-linked and cross-linked ones with a variable density of cross-links [2,3,4,5,6]. Liquid-crystalline epoxy resins belong to a relatively new group of thermosetting materials, and their curing process is not yet completely understood. Their tremendous properties are a cause of interest, so it is crucial to know their behaviour during curing.
One of the first kinetic studies of liquid-crystalline epoxies took place in the early 1990s, when Carfagna et al. [7] carried out their work. Over the next 25 years, there were some other studies on the curing kinetics of this type of thermosetting materials, but there are still blank spaces on the map of LC thermosets knowledge. Work [7] states that there is a possibility of receiving LC phase in thermoset, when the curing process is conducted in a thermal stability range of the resin’s liquid-crystalline phase. Moreover, the activation energy is reduced when polymer is cross-linked in LC phase. Later studies conducted by the same group led by Amendola [8] also showed the formation of LC phase during the curing of 6,6′-bis(2,3-epoxypropoxy)-2,2 binaphthyl with 4,4′-diaminodiphenyl sulfone. Additionally, it was proven that the overall reaction can be modelled and the contribution of the phase transition during curing can be evaluated.
Those conclusions were deeply investigated by different researchers, who studied a wide variety of both resins and hardeners [9,10,11,12,13,14,15]. Different kinetic models provided one almost universal conclusion—the formation of LC phase during curing greatly affects the cross-linking process. There are also some novel studies when the LC phase is present in the curing agent [16, 17] with similar conclusions. Additionally, there was significant improvement in the mechanical properties of these polymers compared to traditional resins. Chen et al. [18] investigated LC thermoset filled with multi-walled carbon nanotubes and found that the lower degree of cure is caused by the restricted movement of polymer chains by the filler, which is the reason for the lack of contact of reactive, functional groups.
The last few years provided new and marvellous opportunities in LC epoxy studies thanks to the evolution of thermal analysis methods and the invention of temperature-modulated DSC (TMDSC), which allows separating reversible and irreversible transformations, which are extremely useful in the case of liquid-crystalline materials because of a lot of overlapping processes with a different nature. With the use of TMDSC, Li and Kessler found that formation of the LC phase led to facilitating the curing reaction and a higher degree of cure [19]. This technique becomes useful in wide variety of studies, concerning investigation in this paper epoxy thermosets [20], but also, for example, amorphization of arsenicals [21], what shows its great potential.
Temperature-modulated DSC has been used before to study the kinetics of the curing process. It has been shown by Hutchinson group that stochastic TMDSC (specifically TOPEM® DSC described in detail in [22], which is a useful, multi-frequency tool for heat capacity measurements—quasi-static, complex, real and imaginary cp as well as for determination of reversing, non-reversing, total, sensible and latent heat flow [23]) provides a great advantage over traditional DSC when it comes to determining the glass transition temperature for a fully cured system of reactive trifunctional epoxy—triglycidyl-p-aminophenol (TGAP) [24]. Other studies suggest that the heat of curing determined by TMDSC may be higher than the corresponding value from DSC; moreover, modulated DSC allows the observation of vitrification–devitrification processes during curing [25, 26]. Complex heat capacity measured by alternating DSC also provides proof of the different reactivity of primary and secondary amines in amine–epoxy systems [27]. Montserrat and Pla suggested, however, that TMDSC has its limitations, especially in epoxy-anhydride compositions, when the required modulation period is too short for this technique [28].
In our work, anisotropic liquid-crystalline epoxy thermosets were synthesized, and the cross-linking process of LC epoxy compositions with aromatic diamine was extensively analysed using the conventional differential scanning calorimetry DSC and TOPEM® temperature-modulated DSC (TMDSC) and nuclear magnetic resonance spectroscopy 1H-NMR. The conventional DSC analysis showed that there is a deformation in the liquid-crystalline transition of LC monomer (i.e. from crystal to liquid-crystalline phase transition) during heating of the analysed composition, which suggested that the partial exothermic cross-linking reaction with epoxy groups’ involvement takes place simultaneously with the endothermic phase transition of liquid-crystalline epoxy monomer (LCEM). In this paper, this hypothesis was confirmed based on the TMDSC and 1H-NMR results.
Experimental
Materials
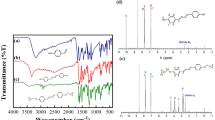
The liquid-crystalline epoxy monomer bis[4-(10,11-epoxyundecanoyloxy)benzoate] p-phenylene (LCEM) used in this study was synthesized and characterized in our laboratory according to a procedure reported in an earlier work [29]. The LCEM monomer exhibits nematic phase between 138 and 183 °C, and during heating, there is a change of its crystalline form at about 80 °C. The 4,4′-diaminodiphenylmethane (DDM, ≥ 97%) used as a curing agent and acetone (p.a.) were purchased from Sigma-Aldrich and were used as received without further purification. The chemical structures and phase transition temperatures of the LCEM monomer and DDM are shown in Fig. 1.
Sample preparation for analysis of curing process
The homogeneous samples of the stoichiometric amounts of LCEM monomer (0.900 g/1.26 mmol) and amine DDM (0.125 g/0.63 mmol) were prepared by shaking the reagents together with 5 mL of acetone. Subsequently, after 5 min of ultrasonication, the solvent was evaporated under reduced pressure at room temperature. Next, the mixture was triturated into fine powder and was stored at 8–10 °C. The so-prepared sample was subjected to DSC and 1H-NMR analyses.
Measurement
DSC
The cross-linking behaviour and thermal properties of reagents and cured samples were studied using differential scanning calorimeter DSC1 from Mettler Toledo with STARe System software. The calibration of the DSC apparatus was carried out using indium and zinc standards supplied by Mettler Toledo. The analysed samples with a mass of about 10 mg were loaded into an aluminium DSC pan and then hermetically sealed with a lid with a small hole. All curves were recorded with an empty aluminium pan as reference, under a nitrogen atmosphere at a flow rate of 50 mL min−1 and at the heating rate of 2, 5, 10 and 20 K min−1, from 0 to 270, 280, 280 and 320 °C accordingly. The DSC analyses with temperature modulation TOPEM® were carried out using the same calorimeter, at heating rate of 2 K min−1, with pulse height of 1 K (±0.500 K) and pulse width of 15–30 s, within temperature range 0–270 °C.
1H-NMR
1H/Proton nuclear magnetic resonance NMR experiments were carried out using a Bruker Avance II Plus spectrometer operating at 500.13 MHz under a static magnetic field of 11.7 T. Spectra were obtained on a spectrometer using standard instrument software (Topspin 1.3) and pulse sequences (zg30), at a probe temperature of 25 °C. The typical acquisition parameters for proton NMR experiments were as follows: acquisition time 3.27 s, spectral width 4000–6000 Hz, nutation angle 30°, relaxation delay 1 s, 32 K data points. 30° single pulse sequence was used for FID accumulation. For all samples studied in this report, a small piece (3–5 mg) was dissolved in 0.6 mL of CD2Cl2 using 5-mm NMR tubes. Chemical shifts were expressed in ppm downfield from the tetramethylsilane (TMS) as internal reference. Deuterated solvents such as DMSO-d6 and CD2Cl2, with deuterium isotope enrichments of 99.6% were purchased from ARMAR Chemicals and Deutero GmbH, respectively.
Results and discussion
Curing behaviour of the LCEM/DDM composition
The composition containing the liquid-crystalline monomer LCEM and amine DDM (Fig. 1) was previously used in our research to obtain ordered polymer networks, with and without selected fillers [30,31,32].
Analysis of the reactivity of LCEM/DDM compositions by the DSC method showed that in the course of heating on the DSC curve some deformation of the endothermic transition peak (from almost Gaussian, symmetrical to unsymmetrical, with irregular shape) of the monomer from the crystalline (Cr2→N) to the liquid-crystalline phase above 130 °C presented itself, what indicates that some more complex process than phase transition is occurring in mentioned temperature and it calls for a deeper investigation. This is shown in Fig. 2, where in addition to the curve of a stoichiometric mixture of LCEM and DDM, the curves of pure components recorded at a heating rate of 5 K min−1 were also presented.
The endothermic changes registered on the curve of the LCEM/DDM composition in the temperature range of 70–92 °C result from the polymorphic transition of the LCEM monomer and the melting of the amine DDM. The difference between areas of DDM melting peak between pure hardener and mixed composition curves is caused by small mass fraction of amine in the composition (about 12%). Enthalpies are calculated in J g−1, so it is expected that DDM melting enthalpy in the mixture with epoxy is about 10 times lower than in hardener alone. The distinct broad exothermic effect accompanying the cross-linking reaction appears above 135 °C, just above the endothermic peak assigned to the melting of the LCEM to the liquid-crystalline nematic phase. Temperature peaks from mixed composition are slightly shifted compared to pure LCEM and hardener, because of mutual interactions. The shape, enthalpy and temperature of the endothermic peak indicate that in this temperature range changes not only related to the phase transition of the monomer occur, and simultaneously, with that transition of the LCEM at about 130 °C, its partial exothermic cross-linking reaction takes place. In order to confirm this hypothesis, subsequent dynamic DSC experiments with different heating rates and modulation TOPEM® and 1H-NMR analyses were performed, wherein especially the thermal effects above 100 °C were analysed by the DSC method. Glass transition temperature is relatively low, what can be caused by long, aliphatic chains in LCER molecule giving elasticizing properties [33]. For better clarity, thermal characteristics of investigated system is presented in Table 1.
Results of the analyses of LCEM/DDM compositions for different heating rates of 2, 5, 10 and 20 K min−1 (Fig. 3) showed that the enthalpy of both transitions: (1) endothermic at about 130 °C and (2) exothermic related to cross-linking above 135 °C increase with increasing heating rate during measurements. The total (summed) thermal effect of that both changes is also increasing, as shown in Fig. 3 and Table 2.
Discrepancies in the enthalpy values between samples heated at different rates may be caused by the appearance or non-appearance of the liquid-crystalline phase during the cross-linking reaction. At work [19], it was found that the formation of the LC phase reduces the viscosity of the reaction mixture, which results in an increase in enthalpy of reaction and degree of curing. However, it is somewhat unexpected that viscosity can have influence not only on the rate of curing, but also on the cross-linking density, and in case of liquid-crystalline epoxies, the presence of mesophase during curing affects the activation energy dependent on the degree of cure, what can be the main cause of this phenomenon [19]. Anisotropic mesophase can also affect spatial possibilities of contacting reactive functional groups, therefore changing the network density. This complicated system of simultaneously occurring factors is the reason why it is almost impossible to accurately estimate the degree of cure. We think, however, that reaction enthalpy 158.1 J g−1 achieved with 20 K min−1 heating rate is close to maximum possible, because of inability to pre-cure in the range of endothermic transition (110–140 °C) caused both by short period of time in case of 20 K min−1 heating (about 1.5 min) and difficulties with LC formation, as presented in [19]. The influence of LC phase formation during heating of the LCEM/DDM composition with different heating rates by using polarized optical microscopy will be the subject of our future studies (Table 2).
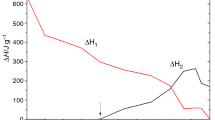
Next, in order to separate the possible reversible effects (e.g. phase transitions of the mixture components) from irreversible (curing the resin), DSC analyses with temperature modulation were performed. The TMDSC curves for the LCEM/DDM composition are shown in Fig. 4. These are heat flow curves marked as non-reversing (red line), reversing (green line) and total heat flow (black line). In Fig. 4, the curve of the composition recorded with the conventional DSC method at a heating rate of 2 K min−1 (blue line) is also presented.
The courses of the total heat flow curves obtained from both DSC analyses, TOPEM® and conventional, are very similar, with similar enthalpy values of endothermic and exothermic phase transitions. On the reversing heat flow curve, the endothermic peak was only recorded, with enthalpy of 19.5 J g−1 higher than the enthalpy of transition in the same temperature range determined from the total heat flow curve. Of particular note is that on the non-reversing heat flow curve two exothermic effects are clearly observed. The enthalpy value of the second peak recorded on that curve above 100 J g−1 coincides with the results from the conventional DSC and total heat flow curve of TMDSC. This clearly confirms the irreversibility of the cross-linking process. However, the presence of the first exothermic effect with maximum at about 125°C and enthalpy of 19 J g−1 suggests a cross-linking reaction at 110–130 °C and reduces the enthalpy of the endothermic peak (49.1 J g−1) on the reversing heat flow curve. The obtained dependences indicate that the total effect of endothermic transformation from a temperature range of 110–130 °C is the sum of the endothermic phase transformation of the monomer and the partial exothermic cross-linking of the LCEM/DDM composition, which was distinctly confirmed by the results of the experiments described below. The difference between shapes of first DSC peak and first total heat flow TOPEM peak may be caused by temperature fluctuations present only in temperature-modulated technique. Moreover, LC transition during hardening should be theoretically visible on reversing heat flow curve affecting the total enthalpy of curing, but we have observed the flat baseline in the curing temperature range. The reason for lack of this reversible transition lies probably in constantly changing and growing of macromolecule during hardening. It is impossible to have reversible effect, when the polymer or pre-polymer chain being the source of LC is no longer present in the composition, because it has transformed into polymer molecule; therefore, the mesophase creation can still be predicted.
Non-reversing results from 70 to 90 °C temperature range did not bring proof of any irreversible process, so this fragment of curve is not presented on the picture for more clarity of crucial part of experiment.
Analysis of pre-cured samples of the LCEM/DDM composition
Two samples were taken from the temperature range of complex thermal changes––meaning that samples of the fresh compositions were heated to 127 and 130 °C in the DSC apparatus with a heating rate of 2 K min−1, and then after rapid cooling, the samples were subjected to further DSC analysis with a heating rate of 10 K min−1 and 1H-NMR analysis. The results of DSC analyses of LCEM/DDM samples, which in the first cycle were heated to 127 and 130 °C and in the second to 270 °C, are shown in Fig. 5 together with the result of the DSC analysis of the fresh mixture.
As was expected, the compositions were heated first to 127 and 130 °C in the next, and second heating cycle was further cross-linking with exothermic effect. Additionally, on the curves from the second run, there are no phase transitions of components. The thermal effect of the cross-linking process is lower than the enthalpy of curing the fresh mixture, and this means that during heating to 127 and 130 °C, a partial cross-linking of the composition occurs.
As already mentioned, to analyse the cross-linking process of the LCEM/DDM system, nuclear magnetic resonance 1H-NMR was also used (Fig. 6). First, spectra of the LCEM and 4,4′-DDM were compared to the spectrum of their fresh stoichiometric mixture (Fig. 6a).
The spectrum of the LCEM/DDM sample allowed stating that the composition is indeed not cured when stored at 8–10 °C, and there were no additional signals compared to pure resin and the hardener. However, the analysis of the LCEM/DDM samples heated up to 127 and 130 °C showed that two chemical shift ranges, where new signals appear, are especially relevant, i.e. 3.9–2.8 ppm (Fig. 6c) and 7.1–6.4 ppm (Fig. 6d). Magnification of this ranges shows confirmation of the earlier results. The signal with a chemical shift of 3.8 ppm (marked in red in Fig. 6c) corresponds to the methine group, and two another with about 3.2 and 2.95 ppm (in blue in Fig. 6c) are split signals from magnetically inequivalent protons of the methylene group created during curing. Both of them are present only after epoxide ring opening, which occurs after reaction with amine. The second chemical shift range 7.1–6.4 ppm shows a similar split of existing signals. Slightly cured resin has in its structure molecules of hardener, with a changed chemical environment compared to pure amine, so new peaks with low intensity are proof of the curing process occurring simultaneously with transition in liquid-crystalline state. Those new signals are a representation of protons marked in green in Fig. 6d, as well as the mentioned peaks. The appearance of additional signals on the 1H-NMR spectra of the LCEM/DDM composition is undeniable proof of partial cross-linking of the compositions heated to 127 and 130 °C.
Conclusions
In this work, the curing reaction of the LCEM monomer with aromatic diamine DDM was studied by the DSC and 1H-NMR analyses. The results of the dynamic conventional DSC and the temperature-modulated TMDSC TOPEM® analyses showed that the exothermic cross-linking process of the investigated composition begins during the endothermic phase transitions of the LCEM monomer. That conclusion was also supported by the results of nuclear magnetic resonance spectroscopy 1H-NMR. The behaviour of the LCEM/DDM composition during heating makes it difficult, e.g. the analysis of the kinetics of non-isothermal curing of that system by the iso-conversional method.
The temperature-modulated DSC method is very useful for optimization of the cross-linking conditions of liquid-crystalline polymer thermosets and the analysis of the polymer compositions that, for example, can require fusion before the proper curing process.
References
Reinitzer F. Beiträge zur Kenntniss des Cholesterins. Monatsh Chem. 1888;9:421–41. https://doi.org/10.1007/BF01516710.
Chung TS. Thermotropic liquid crystal polymers: thin-film polymerization, characterization, blend, and applications. 1st ed. Inc., Lancaster, Pensylwania: Technomic Publishing Company; 2001.
Bhat SG, Ramachandra GS, Bhagavath P, Subrao M, Potukuchi DM, Maddasani S. Self-assembled liquid crystalline materials with fatty acids. J Therm Anal Calorim. 2018;131:989–1000. https://doi.org/10.1007/s10973-017-6879-y.
Fernandes EG, Tombari E, Salvetti G, Galli G, Chiellini E. Dielectric relaxation of thermotropic liquid crystalline polyesters based on α, ω-alkylene-di-4-hydroxybenzoates and 4,4′-alkylenedioxy-dibenzoic acid. J Therm Anal Calorim. 2018;131:627–31. https://doi.org/10.1007/s10973-017-6325-1.
Jape SP, Deshpande VD. Investigation into the morphology, crystallization and melting behaviour of nylon 6,6/LCP blends. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7733-6.
Chandrasekar G, Prabu NPS, Mohan MLN. Calorimetric investigations of hydrogen-bonded liquid crystal binary mixtures. J Therm Anal Calorim. 2018;134:1799–822. https://doi.org/10.1007/s10973-018-7688-7.
Carfagna C, Amendola E, Giamberini M, Filippov AG, Bauer RS. Curing kinetics of liquid-crystalline epoxy resins. Liq Cryst. 1993;13(4):571–84. https://doi.org/10.1080/02678299308026329.
Amendola E, Carfagna C, Giamberini M, Fuccia N, Micco G. Curing reaction kinetics of liquid crystalline resin based on 6,6′-bis (2,3-epoxypropoxy)-2,2′ binaphthyl. Mol Cryst Liq Cryst Sci Technol. 1999;336(1):183–98. https://doi.org/10.1080/10587259908026031.
Liu J, Wang C, Campbell GA, Earls JD, Priester RD. Effects of liquid crystalline structure formation on the curing kinetics of an epoxy resin. J Polym Sci Polym Chem. 1997;35(6):1105–24. https://doi.org/10.1002/(SICI)1099-0518(19970430)35:6%3c1105:AID-POLA14%3e3.0.CO;2-A.
Liu YL, Cai ZQ, Wen X, Pi P, Zheng D, Cheng J, Yang Z. Thermal properties and cure kinetics of a liquid crystalline epoxy resin with biphenyl-aromatic ester mesogen. Thermochim Acta. 2011;513(1–2):88–93. https://doi.org/10.1016/j.tca.2010.11.016.
Cai ZQ, Qi D, Sun J, Ren H, Zhao Q, Zhou Q. Studies on cure kinetics of diglycidyl ether of 4,4′-bisphenol/4,4′-diaminobiphenyl using the advanced isoconversional method. Polym Plast Technol Eng. 2008;47(11):1105–8. https://doi.org/10.1080/03602550802391359.
Balamurugan R, Kannan P. 1,3,4-Oxadiazole epoxy resin-based liquid crystalline thermosets and their cure kinetics. J Mater Sci. 2010;45(5):1321–7. https://doi.org/10.1007/s10853-009-4085-4.
Roşu D, Mititelu A, Caşcaval CN. Cure kinetics of a liquid-crystalline epoxy resin studied by non-isothermal data. Polym Test. 2004;23(2):209–15. https://doi.org/10.1016/S0142-9418(03)00082-5.
Gao J, Huo L, Du Y. Nonisothermal cure kinetics and diffusion effect of liquid-crystalline epoxy sulfonyl bis(1,4-phenylene)bis[4-(2,3-epoxypropyloxy)benzoate] resin with aromatic diamine. J Appl Polym Sci. 2012;125(5):3329–34. https://doi.org/10.1002/app.33877.
Punchaipetch P, Ambrogi V, Giamberini M, Brostow W, Carfagna C, D’Souza N. Epoxy + liquid crystalline epoxy coreacted networks: I. Synthesis and curing kinetics. Polymer. 2001;42(5):2067–75. https://doi.org/10.1016/S0032-3861(00)00505-X.
Wang HM, Zhang YC, Zhu LR, Zhang BL, Zhang YY. Curing behavior and kinetics of epoxy resins cured with liquid crystalline curing agent. J Therm Anal Calorim. 2012;107(3):1205–11. https://doi.org/10.1007/s10973-011-1663-x.
Wang H, Zhang Z, Zhu L, Du Z, Zhang B, Zhang Y. Curing behaviors and kinetics of epoxy resins with a series of biphenyl curing agents having different methylene units. Thermochim Acta. 2011;521(1–2):18–25. https://doi.org/10.1016/j.tca.2011.03.036.
Chen S, Hsu SH, Wu MC, Su WF. Kinetics studies on the accelerated curing of liquid crystalline epoxy resin/multiwalled carbon nanotube nanocomposites. J Polym Sci Polym Phys. 2011;49(4):301–9. https://doi.org/10.1002/polb.22179.
Li Y, Kessler MR. Cure kinetics of liquid crystalline epoxy resins based on biphenyl mesogen. J Therm Anal Calorim. 2014;117(1):481–8. https://doi.org/10.1007/s10973-014-3647-0.
Monteserin C, Blanco M, Laza JM, Aranzabe E, Vilas JL. Thickness effect on the generation of temperature and curing degreegradients in epoxy–amine thermoset systems. J Therm Anal Calorim. 2018;132:1867–81. https://doi.org/10.1007/s10973-018-7062-9.
Shpotyuk O, Kozdras A, Balaz P, Bujnakova Z, Shpotyuk Y. DSC TOPEM® study of high-energy mechanical milling-driven amorphization in b-As4S4-based arsenicals. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7613-0.
Schawe JEK, Hutter T, Heitz C, Alig I, Lellinger D. Stochastic temperature modulation: a new technique in temperature-modulated DSC. Thermochim Acta. 2006;446:147–55. https://doi.org/10.1016/j.tca.2006.01.031.
Wagner M. Thermal analysis in practice collected applications, application handbook. Schwarzenbach: Mettler Toledo; 2009.
Hutchinson JM, Shiravand F, Calventus Y, Fraga I. Isothermal and non-isothermal cure of a tri-functional epoxy resin (TGAP): a stochastic TMDSC study. Thermochim Acta. 2012;529:14–21. https://doi.org/10.1016/j.tca.2011.11.008.
Montserrat S, Martín JG. Non-isothermal curing of a diepoxide-cycloaliphatic diamine system by temperature modulated differential scanning calorimetry. Thermochim Acta. 2002;388(1–2):343–54. https://doi.org/10.1016/S0040-6031(02)00019-9.
Cedeño AJ, Vázquez-Torres H. Kinetic study of the effect of poly(phenyl sulfone) on the curing of an epoxy/amine resin by conventional and by temperature-modulated differential scanning calorimetry. Polym Int. 2005;54(8):1141–52. https://doi.org/10.1002/pi.1818.
Montserrat S, Cima I. Isothermal curing of an epoxy resin by alternating differential scanning calorimetry. Thermochim Acta. 1999;330(1–2):189–200. https://doi.org/10.1016/S0040-6031(99)00033-7.
Montserrat S, Pla X. Use of temperature-modulated DSC in kinetic analysis of a catalysed epoxy-anhydride system. Polym Int. 2004;53(3):326–31. https://doi.org/10.1002/pi.1370.
Galina H, Mossety-Leszczak B. Liquid-crystalline epoxy resins. J Appl Polym Sci. 2007;105(1):224–8. https://doi.org/10.1002/app.26014.
Mossety-Leszczak B, Strachota B, Strachota A, Steinhart M, Šlouf M. The orientation-enhancing effect of diphenyl aluminium phosphate nanorods in a liquid-crystalline epoxy matrix ordered by magnetic field. Eur Polym J. 2015;72:238–55. https://doi.org/10.1016/j.eurpolymj.2015.09.018.
Mossety-Leszczak B, Włodarska M. Liquid-crystalline epoxy thermosets as matrices for ordered nanocomposites: a summary of experimental studies. Polym Compos. 2017;38(2):277–86. https://doi.org/10.1002/pc.23585.
Mossety-Leszczak B, Kisiel M, Szałański P, Włodarska M, Szeluga U, Pusz S. The influence of a magnetic field on the morphology and thermomechanical properties of a liquid crystalline epoxy carbon composite. Polym Compos. 2018;39(S4):2573–83. https://doi.org/10.1002/pc.24848.
Zhou DW, Liang LY, Lu MG. Dimeric liquid crystalline thermosets from azo-containing diglycidyl ether cured by anhydride. Polym Bull. 2011;66(8):1111–23. https://doi.org/10.1007/s00289-010-0401-z.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mossety-Leszczak, B., Kisiel, M., Lechowicz, J.B. et al. Analysis of curing reaction of liquid-crystalline epoxy compositions by using temperature-modulated DSC TOPEM®. J Therm Anal Calorim 138, 2435–2444 (2019). https://doi.org/10.1007/s10973-019-08193-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08193-w