Abstract
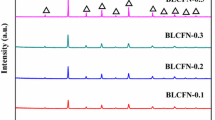
Investigations on Ti, Pr, and Dy substitution and Ti co-substitution with La, Ce, Pr, Nd, and Dy in Y site were carried out. The XRD patterns indicate that the solubility limit for Ti is 5%. The solubility limit for Dy is 100%. Impurities will form if the substituting proportion surpasses the solubility limit for the substituting ion. For the phase composition of all co-substitution samples, only Ti and Dy substituted one is essentially single phase. The largest oxygen content δ reached 1.054, 1.188 and 1.070 for Y0.95Ti0.05BaCo4O7+δ, DyBaCo4O7+δ, Y0.2Ti0.05Dy0.75BaCo4O7+δ, respectively. The largest oxygen content δ reached was 0.900 for Y0.25Pr0.75BaCo2O5+δ.












Similar content being viewed by others
References
Moghtaderi B. Application of chemical looping concept for air separation at high temperatures. Energy Fuels. 2010;24(3):190–8.
Hong J, Chaudhry G, Brisson JG, Field R, Gazzino M, Ahmed FG. Analysis of oxy-fuel combustion power cycle utilizing a pressurized coal combustor. Energy. 2009;34(9):1332–40.
Wang K, Yu QB, Qin Q. The thermodynamic method for selecting oxygen carriers used for chemical looping air separation. J Therm Anal Calorim. 2013;112(2):747–53.
Wang Y, Wang XY, Hua XN, Zhao CC, Wang W. The reduction mechanism and kinetics of Fe2O3 by hydrogen for chemical-looping hydrogen generation. J Therm Anal Calorim. 2017;129(3):1831–8.
Wang BW, Li HY, Ding D, Shen QW, Zhao HB, Zheng CG. Chemical looping combustion characteristics of coal with Fe2O3 oxygen carrier. J Therm Anal Calorim. 2018;132(1):17–27.
Janković B, Dodevski V, Stojmenović M, Krstić S, Popović J. Characterization analysis of raw and pyrolyzed plane tree seed (Platanus orientalis L.) samples for its application in carbon capture and storage (CCS) technology. J Therm Anal Calorim. 2018;133(1):465–80.
Pishahang M, Larring Y, Adánez J, Gáyan P, Sunding M. Fe2O3–Al2O3 oxygen carrier materials for chemical looping combustion, a redox thermodynamic and thermogravimetric evaluation in the presence of H2S. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7422-5.
Ishida M, Yamamoto M, Ohba T. Experimental results of chemical looping combustion with NiO/NiA12O4 particle circulation at 1473 K. Energy Convers Manag. 2002;43(9):1469–78.
Mattisson T, Leion H, Lyngfelt A. Chemical-looping with oxygen uncoupling using CuO/ZrO2 with petroleum coke. Fuel. 2009;88(4):683–90.
Arjmand M, Azad A, Leion H, Lyngfelt A, Mattisson T. Prospects of Al2O3 and MgAl2O4-supported CuO oxygen carriers in chemical-looping combustion (CLC) and chemical-looping with oxygen uncoupling (CLOU). Energy Fuels. 2011;25(11):5493–502.
Shulman A, Cleverstam E, Mattisson T, Lyngfelt A. Manganese/iron, manganese/nickel, and manganese/silicon oxides used in chemical-looping with oxygen uncoupling (CLOU) for combustion of methane. Energy Fuels. 2009;23(10):5269–75.
Azimi G, Leion H, Rydén M, Mattisson T, Lyngfelt A. Investigation of different Mn–Fe oxides as oxygen carrier for chemical-looping with oxygen uncoupling (CLOU). Energy Fuels. 2013;27(1):367–77.
Shulmana A, Cleverstam E, Mattisson T, Lyngfelt A. Chemical-looping with oxygen uncoupling using Mn/Mg-based oxygen carriers-oxygen release and reactivity with methane. Fuel. 2011;90(3):941–50.
Zhang T. Experimental study and analysis of O2–CO2 production based on chemical looping method. MA dissertation, Tsinghua University, 2010.
Shen WQ. The experimental study on performance of O2/CO2 production using perovskite-type oxides. MA dissertation, Huazhong University of Science and Technology, 2011.
Zhang T, Li ZS, Cai NS. Continuous O2–CO2 production using a Co-based oxygen carrier in two parallel fixed-bed reactors. Korean J Chem Eng. 2009;26(3):845–9.
Valldor M, Andersson M. The structure of the new compound YBaCo4O7 with a magnetic feature. Solid State Sci. 2002;4(7):923–31.
Karppinen M, Yanauchi H, Otani S, Fujia T, Motohashi T. Oxygen nonstoichiometry in YBaCo4O7: large low-temperature oxygen absorption/desorption capability. Chem Mater. 2006;18(2):490–4.
Motohashi T, Kadota S, Fjellvag H, Karppinen M, Yamauchi H. Uncommon oxygen intake/release capability of layered cobalt oxides REBaCo4O7+δ: noval oxygen-storage materials. Mater Sci Eng B. 2008;148(1):196–8.
Nagai Y, Yamamoto T, Tanaka T, Yoshida S, Nonaka T, Okamoto T, Suda A, Sugiura M. X-ray absorption fine structure analysis of local structure of CeO2–ZrO2 mixed oxides with the same composition ratio (Ce/Zr = 1). Catal Today. 2002;74(3):225–34.
Kašpar J, Fornasiero P. Nanostructured materials for advanced automotive de-pollution catalysts. Solid State Chem. 2003;171(1–2):19–29.
Hao HS, He QL, Cheng YG, Zhao LM. High-temperature electronic transport properties of (YCa)BaCo4O7 compounds. J Phys Chem Solids. 2014;75(4):495–9.
Zhang SM. Study on the oxygen adsorption/desorption properties of doped-YBaCo4O7 and its oxygen permeation ability. MA dissertation, ZhengZhou University, 2011.
Guo LJ. Study on the oxygen adsorption/desorption properties of YBaCo4O7 and relative compound. MA dissertation, Zhengzhou University, 2005.
Kozeeva LP, Kameneva MY, Lavrov AN, Podberezskaya NV. Synthesis and oxygen behavior of RBaCo4O7+δ (R = Y, Dy–Lu). Inorg Mater. 2013;49(6):626–31.
Parkkima O, Yamauchi H, Karppinen M. Oxygen storage capacity and phase stability of variously substituted YBaCo4O7+δ. Chem Mater. 2013;25(4):599–604.
Martin V. Magnetic investigations on six compounds with the general formula (Ca, Y)Ba(Co, Fe, Al, Zn)4O7 and the structures of YBaCoFeZn2O7 and YBaCo2FeZnO7. Solid State Sci. 2005;7(10):1163–72.
Wang S, Hao HS, Zhu BF, Jia JF, Hu X. Modifying the oxygen adsorption properties of YBaCo4O7 by Ca, Al, and Fe doping. J Mater Sci. 2008;43(15):5385–9.
Räsänen S, Motohashi T, Yamauchi H, Kappinen M. Stability and oxygen-storage characteristics of Al-substituted YBaCo4O7. J Solid State Chem. 2010;183(3):692–5.
Komiyama T, Motohashi T, Masubuchi YJ, Kikkawa S. Synthesis, thermal stability, and oxygen intake/release characteristic of YBa(Co1−xAlx)4O7+δ. Mater Res Bull. 2010;45(10):1527–32.
Frontera C, Caneiro A, Carrillo AE, Oro-Sole J, Garcia-Munoz JL. Tailoring oxygen content on PrBaCo2O5+δ layered cobaltites. Chem Mater. 2005;17(22):5439–45.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 51576035, 51604078), the Fundamental Research Funds for the Central Universities (Grant No. N162504012) and the Post-Doctoral Science Foundation (Grant Nos. 2017M610185, 20170101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Hou, L., Yu, Q., Wang, K. et al. Oxygen storage capacity of substituted YBaCo4O7+δ oxygen carriers. J Therm Anal Calorim 137, 317–325 (2019). https://doi.org/10.1007/s10973-018-7903-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7903-6