Abstract
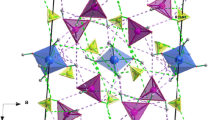
The new crystals of phosphate tellurate family have been grown by slow evaporation at room temperature. The rubidium cesium phosphate tellurate Rb1.84Cs0.16HPO4Te(OH)6 (RbCsPTe) salt is monoclinic with the following unit cell dimensions: a = 8.295(4) Å, b = 7.067(4) Å, c = 12.289(6) Å, β = 90.330(9). The space group is P21/c with Z = 4. The structure was solved by Patterson methods and refined to a final R value of 0.056 for 1822 observed reflections. The RbCsPTe structure contains planes of statistically distributed (\({\text{PO}}_{4}^{3 - }\)) tetrahedra alternating with planes of (\({\text{TeO}}_{6}^{6 - }\)) octahedra, Rb+/Cs+ cations are situated between these planes. The crystalline network is stabilized by mean of hydrogen bonds connecting cations and anions forming a three-dimensional network. Differential scanning calorimetry revealed three-phase transitions at 152, 187 and 207 °C. The thermal analyses (DTA) and (TG) of the title compound have proven that the decomposition of this material starts at a temperature equal to 227 °C. Raman and IR spectra in the frequency range 50–1300 and 500–4000 cm−1, respectively, exhibit the presence and independence of anionic groups in the structure and the importance of the strong hydrogen bonds. The Raman spectroscopy study at various temperatures evinces the presence of the phase transitions revealed in DSC and DTA. The rubidium cesium phosphate tellurate (RbCsPTe) was characterized by impedance spectroscopy technique. Complex impedance measurements are performed on this material as a function of both temperature and frequency. The activation energies obtained from the impedance and modulus spectra are similar, suggesting that the ionic transport in the investigated material can be described by a hopping mechanism.













Similar content being viewed by others
References
Zilber R, Torbjman I, Guitel JC. Structure of sodium sulfate tellurate. Acta Cryst. 1980;B36:2741–3.
Zilber R, Durif A, Averbuch-Pouchot MT. Structure of ammonium sulfate tellurate Te(OH)6·(NH4)2SO4. Acta Cryst. B. 1981;37:650–2.
Zilber R, Durif A, Averbuch-Pouchot MT. Structure of thalium sulfate tellurate Te(OH)6·Tl2SO4. Acta Cryst B. 1982;38:1554–6.
Dammak M, Khemakhem H, Mhiri T. Superprotonic conduction and ferroelectricity in addition cesium sulfate tellurate Cs2SO4·Te(OH)6. J Phys Chem Solids. 2001;62:2069–74.
Dammak M, Khemakhem H, Mhiri T, Kolsi AW, Daoud A. Structural and Vibrational Study of K2SeO4·Te(OH)6 Material. J Solid State Chem. 1999;145:612–8.
Abdelhedi M, Dammak M, Cousson A, Kolsi AW. Structural, calorimetric and conductivity study of the new mixed solution Rb2(SO4)0.5(SeO4)0.5Te(OH)6. J Alloy Compd. 2005;398:55–61.
Durif A, Averbuch-Pouchot MT, Guitel JC. Structures de deux phosphotellurates: Te(OH)6·2(NH4)2HPO4 et Te(OH)6·Na2HPO4·H2O. Acta Cryst. B. 1979;35:1444–7.
Averbuch-Pouchot MT. Structure of potassium sulfate tellurate Te(OH)6·K2SO4. Acta Cryst B. 1989;36:2743–3745.
Averbuch-Pouchot MT. Hydrogen bonding in NH4HSO4·NH4H2PO4. Mater Res Bull. 1981;16:407–11.
Averbuch-Pouchot MT, Durif A, Guitel JC. Structure cristalline d’un phspho-tellurate de rubidium: Te(OH)6·Rb2HPO4·RbH2PO4. Mater Res Bull. 1979;14:1219–23.
Averbuch-Pouchot MT, Durif A, Guitel JC. Crystal structures of two cesium phosphate-tellurates: Te(OH)6·Cs2HPO4 and Te(OH)6·Cs2HPO4·2CsH2PO4. Mater Res Bull. 1980;15:387–95.
Nonius. Kappa CCD Sever Software. The Netherlands: Nonius, BV; 1997.
Betteridge PW, Carruthers JR, Cooper RI, Watkin K. CRYSTALS version 12: software for guided crystal structure analysis. J Appl Cryst. 2003;36:1487.
Brandenburg K, Berndt M. Diamond. Version 2.1.b. Germany: Crystal impact Gb R Bonn; 1999.
Faby J, Loub J, Feltl L. Study of the thermal decompositions of orthotelluric acid, urea and the orthotelluric acid adduct with urea. J Therm Anal. 1982;24:95–100.
Dammak M, Khemakhem H, Mhiri T, Kolsi AW, Daoud A. Structure and characterization of a mixed crystal Rb2SO4·Te(OH)6. J Alloy Compd. 1998;280:107–13.
Dammak M, Mhiri T, Jaud J, Savariault JM. Structural study of the two new caesium sulfate and selenate tellurate Cs2SO4·Te(OH)6 and Cs2SeO4·Te(OH)6. Int J Inorg Mater. 2001;3:861–73.
Bechibani I, Zaafouri A, Dammak M, Ktari L. Investigation of electrical and dielectric properties of the new rubidium arsenate tellurate Rb2HAsO4Te(OH)6 compound. J Alloy Compd. 2017;724:951–8.
Elferjani A, Abdelhedi M, Dammak M, Kolsi AW. Structural, dielectric and vibrational studies of the new mixed solid solution of thallium potassium sulfate selenate tellurate. Appl Phys A. 2016;122:742–54.
Ghorbel K, Litaiem H, Ktari L, Garcia-Granda S, Dammak M. Ionic-protonic conduction analysis and dielectric relaxation behavior of the rubidium ammonium arsenate tellurate. Ionics. 2016;22:251–60.
Frikha H, Abdelhedi M, Dammak M, Garcia-Granda S. Structural single crystal, thermal analysis and vibrational studies of the new rubidium phosphate tellurate Rb2HPO4RbH2PO4Te(OH)6. J. Saudi Chem. Soc. 2017;21:324–33.
Jiao QJ, Zhu YL, Huang H, Ren H. Thermal decomposition of RDX/AP by TG–DSC–MS–FTIR. J Therm Anal Calorim. 2014;116:1125–31.
Frost RL, Locke A, Martens WN. Thermogravimetric analysis of wheatleyite Na2Cu2+(C2O4)2·2H2O. J Therm Anal Calorim. 2008;93:993–7.
Chabchoub N, Khemakhem H, Gargouri M. Ferroelectricity and superionic conduction in telluric sulfates MM′(SO4)Te(OH)6 (M, M′ = K, Rb and Cs). J Alloy Compd. 2003;359:84–90.
Philip D, Abraham D, Aruldhas G. IR and single-crystal Raman spectra of Te(OH)6·2CO(NH2)2. J Raman Spectrosc. 1990;21:521–2.
Philip D, Aruldhas G. Vibrational spectra of thallium and rubidium phosphotellurates. J Raman Spectrosc. 1989;20:637–8.
Viswanathan K, Nayar VU, Aruldhas G. Vibrational spectra of cesium tellurate phosphate. J Infrared Phys. 1986;26:353–6.
Gaumt J. The infra-red spectra and molecular structure of some group 6 hexafluoride. Trans Faraday Soc. 1953;49:1122–31.
Siebert H. Infra-red spectrum of telluric, tellurates and antimonatcs. J Chem. 1959;301:161–70.
Farmer VC (Ed). The infrared spectra of minerals. The Mineralogical Society. 1974;539.
Bauerle JE. Study of solid electrolyte polarization by a complex admittance method. J Phys Chem. 1969;30:2657–70.
Boudaya C, Chabchoub N, Khemakhem H, Von der Mühll R. Ionic conduction and dielectric properties in the telluric sulfate K(SO)4Te(OH)6. J Alloy Compd. 2003;352:304–8.
Dammak M, Khemakhem H, Zouari N, Kolsi AW, Mhiri T. Electrical properties of ferroelectric addition compound K2SeO4.Te(OH)6. Solid State Ionics. 2000;127:125–32.
Masmoudi W, Kamoun S, Gargouri M. AC conductivity and dielectric studies of (C5H10N)2BiCl5 compound. Ionics. 2012;18:117–26.
Anantha PS, Hariharn K. Ac conductivity analysis and dielectric relaxation behaviour of NaNO3–Al2O3 composites. Mater Sci Eng. 2005;B121:12–9.
Kyritsis A, Pissi P, Grammatikakis J. Dielectric relaxation spectroscopy in poly (hydroxyethyl acrylates)/water hydrogels. J Polym Sci Phys. 1995;33:1737–50.
Cao W, Gerhardt R. Calculation of various relaxation times and conductivity for a single dielectric relaxation process. Solid State Ionics. 1990;42:213–21.
Chowdari BVR, Gopalakrishnan R. AC conductivity analysis of glassy silver iodomolybdate system. Solid State Ionics. 1987;23:225–33.
Acknowledgements
This work is supported by the Ministry of Superior Education and Research of Tunisia. All the authors would like to express their thanks to Pr. H. Khemakhem for his help in the spectroscopic Raman measurements. All the authors would like to express their thanks to Pr. A. Ben Salah for his help in the X-ray diffraction measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Frikha, H., Abdelhedi, M., Louati, B. et al. Structure, thermal decomposition, vibrational and impedance spectroscopy studies of an rubidium cesium phosphate tellurate Rb1.84Cs0.16 HPO4Te(OH)6 . J Therm Anal Calorim 131, 2795–2808 (2018). https://doi.org/10.1007/s10973-017-6796-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6796-0