Abstract
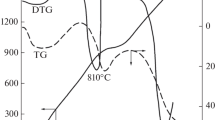
Dissociation of hematite is an undesirable reaction for iron ore pelletizing process leading to severe deterioration in compressive cold strength and reducibility factors. It was shown that raising temperature in an induration machine would cause hematite’s dissociation, which is either present in the primary ore or formed by oxidation of magnetite in the feed. The oxidation reaction of magnetite is exothermic, which complicates temperature control within the non-isothermal area of preheating. Kinetics of the dissociation reaction is the temperature’s primary function, which controls the extent of the reaction. Pure hematite samples were subjected to several runs of thermal analysis carried out under both air and inert atmosphere, in order to achieve a comprehensive knowledge about the temperature dependencies of dissociation kinetics. Due to the observed uniformity, isoconversional methods were chosen in the present work over isothermal and non-isothermal for calculation of kinetic parameters of the reaction. Respectively, activation energy values of hematite dissociation were found to be 324 and 382 kJ mol−1 in inert and air atmosphere. The high value of activation energy implies strong dependency of the single-step reaction rate on the temperature. It was also observed that the forward reaction had higher activation energy than the backward reaction; hence, an increase in temperature results in an overall acceleration of the dissociation reaction.









Similar content being viewed by others
References
Barati M. Dynamic simulation of pellet induration process in straight-grate system. Int J Miner Process. 2008;89(1):30–9.
Yurev BP, Spirin NA. Oxidation of iron ore pellets. Steel Trans. 2011;41:400–3.
Forsmo SPE. Influence of green pellet properties on pelletizing of magnetite iron ore. Lulea: Lulea University of Technology; 2007.
Li G, Jiang T, Zhang Y. Recrystallization of Fe2O3 during the induration of iron ore oxidation pellets. In: Sztwiertnia K, editor. Recrystallization. Shanghai: InTech Open Science Publishing; 2012. pp. 329–350 (chapter 13).
Meyer K. Pelletizing of iron ores. Berlin: Springer; 1980.
Qu Y, Yang Y, Zou Z, Zeilstra C, Meijer K, Boom R. Thermal decomposition behaviour of fine iron ore particles. ISIJ Int. 2014;54(10):2196–205.
Sorescu M, Xu T. Particle size effects on the thermal behavior of hematite. J Therm Anal Calorim. 2012;107(2):463–9.
Darken L, Gurry R. The system iron—oxygen. II. Equilibrium and thermodynamics of liquid oxide and other phases. J Am Chem Soc. 1946;68(5):798–816.
Ferreira S, Siguin D, Garcia F. Thermal analysis of sintering of magnetite pellets. Ironmak Steelmak. 1994;21(2):119–23.
Budrugeac P. Differential non-linear isoconversional procedure for evaluating the activation energy of non-isothermal reactions. J Therm Anal Calorim. 2002;68(1):131–9.
Ebrahimi-Kahrizsangi R, Abbasi MH. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans Nonferr Met Soc China. 2008;18(1):217–21. doi:10.1016/S1003-6326(08)60039-4.
Vyazovkin S, Wight CA. Isothermal and non-isothermal kinetics of thermally activated reactions of solids. Int Rev Phys Chem. 1998;17(3):407–33.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S, Chrissafis K, Koga N, Lorenzo MLD, Pijolet M, Roduit B, et al. ICTAC kinetics committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Maciejewski M. Computational aspects of kinetic analysis. Part B: the ICTAC kinetics project—the decomposition kinetics of calcium carbonate revisited, or some tips on survival in the kinetic minefield. Thermochim Acta. 2000;355(1):145–54.
Vyazovkin S. Isoconversional kinetics of thermally stimulated processes. New York: Springer; 2015.
Gaviria JP, Bohe A, Pasquevich A, Pasquevich DM. Hematite to magnetite reduction monitored by mossbaur spectroscopy and X-ray diffraction. Physica. 2007;389:198–201.
Piotrowski K, Mondal K, Wiltowski T, Dydo P, Rizeg G. Topochemical approach of kinetics of the reduction of hematite to wustite. Chem Eng J. 2007;131:73–82.
ASTM. E1641: standard test method for decomposition kinetics by thermogravimetry using the Ozawa/Flynn/Wall method. West Conshohocken, PA: ASTM International; 2015.
ASTM. E698: standard test method for arrhenius kinetic constants for thermally unstable materials using differential scanning calorimetry and the Flynn/Wall/Ozawa method. West Conshohocken, PA: ASTM International; 2011.
House JE. Principles of chemical kinetics. 2nd ed. Burlington: Academic press; 2007.
Acknowledgements
This work was performed under financial support of Gol-e-Gohar mining and industrial company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salmani, M., Alamdari, E.K. & Firoozi, S. Isoconversional analysis of thermal dissociation kinetics of hematite in air and inert atmospheres. J Therm Anal Calorim 128, 1385–1390 (2017). https://doi.org/10.1007/s10973-016-5981-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5981-x