Abstract
In the paper, the results of investigation concerning the influence of 0.3% addition of hydroxypropyl methylcellulose (MC) of 40 and 70 Pa s viscosity on the hydration process of main clinker phases C3S and C3A are presented. The course of hydration is documented using microcalorimetry, and formed phases were identified by XRD. The research indicated that methylcellulose inhibited the hydration process of clinker phases, analysed both, separately and in their mixture. Moreover, it was found that the presence of MC admixture also led to the reduction in calcium sulphate dihydrate reactivity in the system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In cement mortars, there is usually very little addition of methylcellulose used. Such little amount is enough to obtain several desired effects—advantageous modifications of mortars. However, the main effects of MC action—modification of: rheology, water demand, bond strength to substrate—are accompanied by several side effects like modification of: microstructure, porosity, hydration course, setting and hardening of mortars. Without the doubt, a modification of hydration and setting processes of cementitious binder should be included in the areas especially sensitive. Generally, the mechanism according to which MC retards the hydration and delays the setting of cement can be associated with: adsorption of admixture on the surface of binder particles or on hydration products, decrease in ions mobility, impediments in the process of nucleation or growth of crystals, complexation of constituents of reacting system, formation of membranes on the particles of hydrating cement [1]. Most probably a combination of many factors mentioned above influences the hydration course, and nevertheless a search for the main action effect is reported in the literature. In the calorimetric studies [2, 3], it is indicated that the delay and the extension of hydration processes only concerns the induction and acceleration periods of hydration; important changes of rheological properties and porosity of prepared mortar are taking place independently from delayed hydration due to the agglomeration of suspended particles and air bubbles [2, 4].
The influence of methylcellulose on the hydration of cement is concentrated on the first hours of process, when the correlation between delay of setting and MC adsorption on the surface of hydration products is observed [5]. The mechanism of methylcellulose adsorption—rather on the hydration products than on the binder constituents—becomes the most important factor affecting the hydration kinetics of cementitious—organic systems in the model investigations with the use of pure clinker phases [6–10]. The observations included in the cited literature show wide spectrum of possible interactions—from reconstruction of layer structure of gel hydrates on the C3S surface, through adsorption on the hydrates surface-calcium hydroxide and hydroaluminates (in the latter case without influence on the microstructure of hydration products) up to the agreeably noted practical lack of adsorption on ettringite surface [6, 8].
Performed up to now, model experiments of phase composition of cement matrix and properties of hardened cement mortars with different additions of methylcellulose of different viscosity have indicated that hydration process of cement phases is essentially modified [9–12]. Despite low methylcellulose addition, equal to about 0.3% by phase mass, these changes are high. They are mainly characterized by maintaining substantial gypsum content in the paste, practically until the end of hydration process [11, 12].
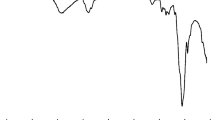
The calorimetric curve of tricalcium aluminate hydration is presented in Fig. 1 [10].
Microcalorimetric curve of C3A hydration in water [10]
The reaction course of tricalcium silicate with water is well presented by microcalorimetric curve obtained at isothermal conditions (Fig. 2) [13].
Microcalorimetric curve of C3S hydration [13]
Influence of admixtures on the reaction of tricalcium silicate with water can be easily investigated by calorimetric measurements, because they allow to evaluate the changes of induction period as well as the changes of course of main maximum indicating the rate of that process [14]. Therefore, this method has been also used in the study.
Materials and methods
Experiments have been performed aiming to explain the influence of methylcellulose on the hydration process, both C3A and C3S phases as well as their mixture in the proportion of 6:1, in which the following materials have been used:
-
synthetic alite (C3S),
-
synthetic tricalcium aluminate (C3A),
-
gypsum (CŜ),
-
methylcellulose of plastic viscosity equal to 40 and 70 Pa s (MC-40 and MC-70).
Alite used in the study was synthesized in a Kanthal furnace at the temperature of 1400 °C. Mixture of calcium hydroxide (pure for analysis) and silica (Aerosil from Degussa) was used as raw materials. Moreover, little amounts of alumina hydroxide and magnesium hydroxide were added, ensuring their participation in the form of oxides at the level of about 1%. XRD pattern of alite indicated monoclinic symmetry T2 [14, 15] and slight content of belite. It was decided to use alite sample of such composition in the study, because slight content of belite would not influence the reaction of alite with water.
Tricalcium aluminate used in the study was synthesized in a Kanthal furnace at the temperature of 1400 °C. Mixture of calcium hydroxide and aluminium hydroxide (pure for analysis) was used as raw materials. In the XRD pattern of tricalcium aluminate, no other phases were identified.
Gypsum came from the desulphurization of flue gases and did not contain other admixtures detectable by XRD.
Methylcellulose used in the experiments was a cellulose ethers without any admixtures like starch ethers, polyesters or acrylates. The viscosity is given by the manufacturer’s declaration (for 2% water solution).
Distilled water was used for tests.
From the materials listed above, eighteen samples of composition presented below have been prepared:
High addition of gypsum was used, equal to 9 and 10% by mass, because in the cement paste the ratio of that was constituent also high.
The samples were prepared firstly by blending dry constituents, and the mixture was stored in tightly sealed polyethylene containers. Subsequently, distilled water was added at constant water-to-mixture ratio, equal to 0.5. The hydration was carried on at room temperature of 22 °C ± 1°. The hydration process was interrupted after 1, 3, 6, 12, 24, 36, 48 and 168 h by washing the samples with absolute ethyl alcohol. Then, in order to precisely evaporate alcohol, the samples were dried for 2 h at temperature of 40 °C.
Mineral composition of studied materials was examined by X-ray diffraction method, using diffractometer system X’PertPro MPD PANalytical (anode material Cu, generator settings: anode current 35 mA, voltage 45 kV, start–end positions 7°–60° 2θ). The analysed samples were finely ground and homogenized, and thereafter, the measurement was carried out. This method is rapid analytical technique used for phase identification of a crystalline material. XRD measurements was analysed with HighScore Plus program.
The nonisothermal–nonadiabatic differential microcalorimeter was used to follow the heat evolution on hydration (modified BMR differential microcalorimeter from the Institute of Physical Chemistry, Polish Academy of Science in Warsaw). Hydrating pastes were prepared by mixing of 5 g samples with 2.5 mL of water (water-to-solid ratio 0.5; as in many standard measurements for cements); the initial temperature was kept constant at 25 °C. The heat evolved values were measured with accuracy of ±5 J g−1.
Results
Microcalorimetric analysis of tricalcium aluminate hydration
The course of hydration process of C3A sample in water at room temperature is typical, complying with the literature data and corresponding to standard setting, and is presented in Fig. 3.
After rapid reaction with water, weakly marked induction period appears which is associated with the formation of hexagonal hydrates layer, in this case C4AH13, which inhibits further hydration process by formation of an impermeable barrier layer.
Substantial delay of hydration process of C3A caused by MC-70 addition is visible on the microcalorimetric curve. The induction period is extended up to about 48 h of hydration and its end is faintly marked. However, after about 70 h, a sudden acceleration of reaction appears, lasting shortly. It is connected with essential increase in heat evolution rate. The retardation of C3A reaction with water is very well noticeable in the total amount of heat evolved value, after different hydration time, as it is presented in Table 1. It is slightly higher in the case of methylcellulose of higher viscosity.
Microcalorimetric curves of C3A hydration in the mixture with gypsum and MC-70 have analogical course to the curves obtained by Jawed et al. [14] for tricalcium aluminate without gypsum, but of delayed hydration. They are characterized by short induction period followed by second maximum associated with crystallization of hexagonal hydrate. Higher reaction delay in the case of MC-70 is marked by significantly lower thermal effect in the area of the main maximum. In addition, the induction period is slightly extended, as given in Table 2.
Microcalorimetric analysis of tricalcium silicate hydration
As indicated by the results given in Table 3, the delay of alite reaction with water is the highest in the case of addition of methylcellulose only. Differences in the viscosity between two types of methylcellulose—40 and 70 Pa s—practically have no influence. The retardation caused by methylcellulose addition in the samples with gypsum is significantly weaker, and however, in the period up to 24 h, it is significantly higher in the case of methylcellulose of higher plastic viscosity. After 48 h, there is no more difference between two types of methylcellulose. It cannot be excluded, as it has been found in C3A case, that methylcellulose particles are adsorbed on the gypsum crystals.
The retardation caused by gypsum addition reaches 12 h, while after 36 h it is less visible. After 72 h, the heat of hydration is already higher than in the case of sample with gypsum. Such unexpected influence of gypsum needs further examination. Initially, it can be hypothesized that due to the calcium ions present in alite, a layer of hydrates is formed on surface that layer inhibits water access to C3S. The information given in the literature concerns advantageous influence of gypsum, generally sulphate ions, on the hydration of alite in the case of cement, in which C3A is present [16]. C3A completely changes the conditions of sulphate ions effect on the reaction of alite with water.
The delay of hydration process of C3S caused by addition of gypsum and MC-70 is noticeable on the microcalorimetric curves (Fig. 4). The induction period is somewhat extended, and a main maximum is significantly decreased and retarded. This decrease is the highest in the samples with addition of methylcellulose only, while in the case of simultaneous addition of gypsum and MC it is significantly less decreased.
Microcalorimetric examinations of alite and C3A mixture with addition of gypsum and methylcellulose
The course of calorimetric curve presenting a rate of heat evolution is shown in Fig. 5.
The curve for alite + tricalcium aluminate system has two characteristic maxima. First one, less intensive, occurring in the early hours of hydration, followed by induction period of reaction which is finished with a rapid increase in amount of evolved heat after about 12 h of process and the specific maximum after 28 h of reaction. Similar effect is observed for chosen range of mixtures of Portland cement and alumina cement [17].
According to the literature data, the addition of CaSO4 × 2H2O to the mixture of tricalcium silicate and tricalcium aluminate accelerates the process of heat evolution. In this case, the maximum occurs after 19.5 h. However, it indicates slightly lower intensity than in the case of system without gypsum addition.
The addition of MC-70 to C3S + C3A mixture resulted in an extension of induction period and significant decrease in maximum intensity. Maximum of heat evolution rate appeared only after about 88 h. Simultaneously, the induction period has been extended up to about 40 h since initiation of reaction, what is consistent with the results of XRD investigations.
Combination of gypsum and MC-70 addition also brings about a shift of maximum towards later hours of reaction; however, it appears after 45 h, so in time almost two times shorter than in the case of addition of methylcellulose only. The intensity of maximum is equal to the maximum for addition of MC-70.
After addition of methylcellulose of lower plastic viscosity, there is practically no difference in the course of calorimetric curves.
The amount of evolved heat during hydration time of discussed systems is presented in Table 4.
XRD analysis of alite and tricalcium aluminate mixture
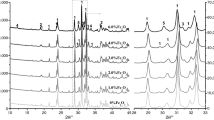
For documenting of processes in the multicomponent systems, the diffraction patterns of pastes after 24 h of hydration are shown in Figs. 6–8.
Figure 6 shows a situation where the hydration of C3S is blocked by hydroaluminates. Hydration of tricalcium aluminate is quite advanced, but only small peaks of portlandite (from alite hydration) can be found after around 24 h of process. The MC presence in such a system practically does not change quantitative relations.
The introduction of gypsum (Fig. 7) brings about the transformation of AFM to AFT (ettringite), resulting in the accelerated hydration of alite and to a lesser degree the hydration of tricalcium aluminate. Viscosity change caused by the introduction of the MC or its possible adsorption does not affect the C3A conversion to ettringite (Fig. 8). A significant reduction in the amount of portlandite that is the slowing down the hydration of alite is observed.
Conclusions
The results of calorimetric measurements are well compatible with the data reported [9–11], dealing with the assessment of hydration kinetics by XRD method.
Methylcellulose has a strong delaying impact on the hydration of C3S, irrespectively of the rheological characteristics of this admixture. This effect is similar as in the presence of gypsum addition, acting through the adsorption on the surface of C3S grains. However, at simultaneous addition of methylcellulose and gypsum, the hydration is not further retarded (the induction period is not prolonged) but the total heat effect is reduced. This retardation is more pronounced at higher viscosity of MC.
Methylcellulose has a strong delaying impact on the hydration of C3A with no additives, irrespectively of the rheological characteristics of this admixture, and however, as the hydration of C3A with gypsum is considered, this retardation increases at higher viscosity of MC because of the limited diffusion of ions through the viscous solution.
The heat evolution in the hydrating C3S + C3A systems is accelerated by gypsum addition (shorter induction period) with simultaneous reduction in total heat evolved. This accelerating effect of gypsum is visible as the systems with MC, having retarding impact on C3S + C3A hydration, are considered.
References
Knapen E, Van Gemert D. Cement hydration and microstructure formation in the presence of water-soluble polymers. Cement Concrete Res. 2009;39:6–13.
Betioli AM, Gleize PJP, Silva DA, John VM, Pileggi RG. Effect of HMEC on the consolidation of cement pastes: isothermal calorimetry versus oscillatory rheometry. Cement Concrete Res. 2009;39:440–5.
Zhang G, Zhao J, Wang P, et al. Effect of HEMC on the early hydration of Portland cement highlighted by isothermal calorimetry. J Therm Anal Calorim. 2015;119:1833–43.
Patural L, Marchal P, Govin A, Grosseau P, Ruot B, Devès O. Cellulose ethers influence on water retention and consistency in cement-based mortars. Cement Concrete Res. 2011;41:46–55.
Pourchez J, Grosseau P, Ruot B. Changes in C3S hydration in the presence of cellulose ethers. Cement Concrete Res. 2010;40:179–88.
Pourchez J, Grosseau P, Ruot B. Current understanding of cellulose ethers impact on the hydration of C3A and C3A-sulphate systems. Cement Concrete Res. 2009;39:664–9.
Silva DA, Monteiro PJM. The influence of polymers on the hydration of Portland cement phases analyzed by soft X-ray transmission microscopy. Cement Concrete Res. 2006;36:1501–7.
Müller I. Influence of cellulose ethers on the kinetics of early Portland cement hydration. Ph.D. Thesis. Münster: Westfälische Wilhelms-Universität;2006.
Pichniarczyk P. The influence of methylcellulose on hydration of tricalcium aluminate. Cement Wapno Beton. 2013;2:35–9.
Pichniarczyk P. The influence of methylcellulose on the hydration process of alite and tricalcium aluminate mixture. Cement Wapno Beton. 2014;6:405–15.
Pichniarczyk P. The influence of methylcellulose on alite hydration process. Cement Wapno Beton. 2013;4:245–55.
Pichniarczyk P, Niziurska P. Properties of ceramic tile adhesives modified by different viscosity hydroxypropyl methylcellulose. Constr Build Mater. 2015;77:227–32.
Kurdowski W. Chemia cementu i betonu. Warszawa: PWN; 2010.
Jawed I, Skalny J, Young JF. Hydration of Portland Cement. In: Barnes P, editor. Structure & Performance of Cements. London: Applied Science Publishers; 1983. p. 237–317.
Maki I. Relationship of processing parameters to clinker properties; influence of minor components. Proc. 8th ICCC, Rio de Janeiro; 1986. vol. 1, p. 34–47.
Guinier A. Regourd M. Structure of portland cement minerals. Proc. 5th ICCC, Tokyo, Japan; 1968. vol. 1, p. 1–41.
Gawlicki M, Nocuń-Wczelik W, Bąk Ł. Calorimetry in the studies of cement hydration setting and hardening of Portland cement–calcium aluminate cement mixtures. J Therm Anal Calorim. 2010;100:571–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pichniarczyk, P., Malata, G. Microcalorimetric analysis of methylcellulose influence on the hydration process of tricalcium aluminate, alite and their mixture. J Therm Anal Calorim 128, 771–778 (2017). https://doi.org/10.1007/s10973-016-5960-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5960-2