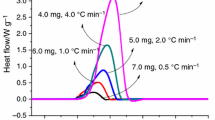
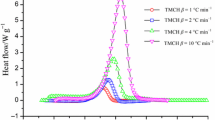
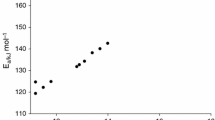
Abstract
Thermal stability parameters were evaluated for the decomposition reaction of tert-butyl peroxy-3,5,5-trimethylhexanoate (TBPMH), a free-radical initiator, by differential scanning calorimetry. According to the results, the apparent exothermic onset temperature, heat of decomposition, and time to maximum rate under adiabatic conditions of TBPMH were 103.0 °C, −924.0 kJ mol−1, and 9.81 min (at 90.0 °C), respectively. A kinetic model was also established under different nonisothermal conditions to evaluate the kinetic behavior of TBPMH by model-free technique and model-fitting method. The self-accelerating decomposition temperature (SADT) was calculated and was similar to that reported in the literature; the SADT values corresponding to various package sizes were also calculated, and an increase in mass caused a drop in SADT. These results can provide a solution to prevent runaway reactions during the storage and transportation of TBPMH, and the applied technique can be a substitute for the complicated procedures and large-scale experiments inherent in traditional analysis methods, thereby preventing process thermal accidents, environmental pollution, and energy depletion.







Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor (s−1)
- C :
-
Constant number (dimensionless)
- C p :
-
Specific heat capacity (J g−1 K−1)
- C p,c :
-
Test cell-specific heat capacity (J g−1 K−1)
- C p,s :
-
Sample-specific heat capacity (J g−1 K−1)
- dα/dt :
-
Conversion rate (s−1)
- E a :
-
Apparent activation energy (kj mol−1)
- f(α):
-
Function of degree of conversion (dimensionless)
- g :
-
Geometry factor (dimensionless)
- k :
-
Reaction rate constant (dimensionless)
- M c :
-
Test cell mass (mg)
- M s :
-
Sample mass (mg)
- MTSR:
-
Maximum temperature of synthesis reaction (°C)
- N :
-
Reaction order (dimensionless)
- n i :
-
Reaction order of ith stage (dimensionless)
- r :
-
Correlation coefficient, −1.0 to 1.0 (dimensionless)
- R :
-
Gas constant (8.314 J K−1 mol−1)
- R 2 :
-
Coefficient of determination 0.0–1.0 (dimensionless)
- SADT:
-
Self-accelerating decomposition temperature (°C)
- T :
-
Absolute temperature (K)
- T 0 :
-
Apparent exothermic onset temperature (°C)
- T f :
-
Final temperature (°C)
- T max :
-
Maximum reaction temperature (°C)
- TMRad :
-
Time to maximum rate under adiabatic conditions (min)
- T P :
-
Peak temperature (°C)
- T :
-
Time (s)
- x :
-
Radius of package (m)
- y(α):
-
Function to define the kinetic model for the model-fitting method (dimensionless)
- z :
-
Autocatalytic constant (dimensionless)
- α :
-
Degree of conversion (dimensionless)
- α max :
-
Maximum point at function y(α) (dimensionless)
- α p :
-
Maximum degree of conversion at specific heat flow (dimensionless)
- β :
-
Heating rate (°C min−1)
- ρ :
-
Density (kg m−3)
- λ :
-
Thermal conductivity (W m−1 K−1)
- Φ :
-
Thermal inertia (dimensionless)
- ∆H d :
-
Heat of decomposition (J g−1)
- ∆T ad :
-
Adiabatic temperature rise (°C)
References
Talouba IB, Balland L, Mouhab N, Chang CT, Abdelghani-Idrissi MA. Kinetic parameter estimation for decomposition of organic peroxides by means of DSC measurements. J Loss Prevent Proc Ind. 2011;24:391–6.
Sanchirico R. Adiabatic behavior of thermal unstable compounds evaluated by means of dynamic scanning calorimetric (DSC) techniques. AIChE J. 2013;59:3806–15.
Hou HY, Shu CM, Duh YS. Exothermic decomposition of cumene hydroperoxide at low temperature conditions. AIChE J. 2011;47:1893–6.
Westerterp KR, Molga EJ. Safety and runaway prevention in batch and semibatch reactors—a review. Chem Eng Res Des. 2006;84:543–52.
Yan QL, Zeman S, Jiménez PES, Zhao FQ, Pérez-Maqueda LA, Málek J. The effect of polymer matrices on the thermal hazard properties of RDX-based PBXs by using model-free and combined kinetic analysis. J Hazard Mater. 2014;271:185–95.
Duh YS, Wu XH, Kao CS. Hazard ratings for organic peroxides. Process Saf Prog. 2008;27:89–99.
Liu SH, Hou HY, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids and alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;566:226–32.
You ML, Liu MY, Wu SH, Chi JH, Shu CM. Thermal explosion and runaway reaction simulation of lauroyl peroxide by DSC tests. J Therm Anal Calorim. 2009;96:777–82.
Safety and handling of organic peroxides, The Society of the Plastics Industry, USA; 2016. http://www.plasticsindustry.org/.
Lin CP, Chang CP, Chou YC, Shu CM. Modeling solid thermal explosion containment on reactor HNIW and HMX. J Hazard Mater. 2010;176:549–58.
Copelli S, Derudi M, Cattaneo CS, Nano G, Raboni M, Torretta V, Rota R. Synthesis of 4-Chloro-3-nitrobenzotrifluoride: industrial thermal runaway simulation due to cooling system failure. Process Saf Environ Prot. 2014;92:659–68.
Safety Data Sheet, Akzo Nobel base chemicals BV, The Netherlands (2015). http://www.akzonobel.com/.
Safety Data Sheet, Alibaba Group. China (2016). http://www.alibaba.com/showroom/tert–butyl-peroxy–3-5-5–trimethylhexanoate-tbpmh.html.
Tong JW, Chen WC, Tsai YT, Cao Y, Chen JR, Shu CM. Incompatible reaction for (3-4-epoxycyclohexane) methyl-3′-4′-epoxycyclohexyl-carboxylate (EEC) by calorimetric technology and theoretical kinetic model. J Therm Anal Calorim. 2014;116:1445–52.
STARe Software with Solaris Operating System. Operating instructions. Sweden: Mettler Toledo; 2015.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Montserrat S, Málek J, Colomer P. Thermal degradation kinetics of epoxy-anhydride resins: I. Influence of a silica filler. Thermochim Acta. 1998;313:83–95.
Yoo MJ, Kim SH, Park SD, Lee WS, Sun JW, Choi JH, Nahm S. Investigation of curing kinetics of various cycloaliphatic epoxy resins using dynamic thermal analysis. Eur Polym J. 2010;46:1158–62.
Málek J. The applicability of Johnson-Mehl-Avrami model in the thermal analysis of the crystallization kinetics of glasses. Thermochim Acta. 1995;267:61–73.
Shah S, Fischer U, Hungerbühler K. A hierarchical approach for the evaluation of chemical process aspects from the perspective of inherent safety. Process Saf Environ Prot. 2003;81:430–43.
Adnađević B, Janković B, Kolar-Anić LJ, Minić D. Normalized Weibull distribution function for modelling the kinetics of non-isothermal dehydration of equilibrium swollen poly(acrylic acid) hydrogel. Chem Eng J. 2007;130:11–7.
Singh H, Chavda A, Nandula S, Jasra RV, Maiti M. Kinetic study on stereospecific polymerization of 1,3-butadiene using a nickel based catalyst system in environmentally friendly solvent. Ind Eng Chem Res. 2012;51:11066–71.
Tsai YT, You ML, Qian XM, Shu CM. Calorimetric techniques combined with various thermokinetic models to evaluate incompatible hazard of tert-butyl peroxy-2-ethyl hexanoate mixed with metal ions. Ind Eng Chem Res. 2013;52:8206–15.
Saraf SR, Rogers WJ, Mannan MS. Prediction of reactive hazards based on molecular structure. J Hazard Mater. 2003;99:15–29.
Xiao HM, Ma XQ, Lai ZY. Isoconversional kinetic analysis of co-combustion of sewage sludge with straw and coal. Appl Energ. 2009;86:1741–5.
Kozlowski C, Kurko K. Consideration of autocatalytic behaviour in determination of self-accelerating decomposition temperature. Burr Ridge: Fauske and Associates; 2008.
Fauske HK. Gassy system vent sizing the role of two-phase flow. Burr Ridge: Fauske and Associates; 2011.
Lu G, Zhang C, Chen L, Chen W, Yang T, Zhou Y. Kinetic analysis and self-accelerating decomposition temperature (SADT) of β-nitroso-α-naphthol. Process Saf Environ Prot. 2015;96:69–76.
Malow M, Wehrstedt KD. Prediction of the self–accelerating decomposition temperature (SADT) for liquid organic peroxides from differential scanning calorimetry (DSC) measurements. J Hazard Mater. 2005;120:21–4.
Sato Y, Okada K, Akiyoshi M, Murayama S, Matsunaga T. Diphenylmethane diisocyanate self-polymerization: thermal hazard evaluation and proof of runaway reaction in gram scale. J Loss Prevent Proc Ind. 2011;24:558–62.
Tsai LC, Tsai YT, Lin CP, Liu SL, Wu TC, Shu CM. Isothermal versus non-isothermal calorimetric technique to evaluate thermokinetic parameters and thermal hazard of tert-butyl peroxy-2-ethyl hexanoate. J Therm Anal Calorim. 2012;109:1291–6.
Naranjo RA, Conesa JA, Pedretti EF, Romero OR. Kinetic analysis: simultaneous modelling of pyrolysis and combustion processes of dichrostachys cinerea. Biomass Bioenerg. 2012;36:170–5.
Chi JH, Wu SH, Charpentier JC, Yet-Pole I, Shu CM. Thermal hazard accident investigation of hydrogen peroxide mixing with propanone employing calorimetric approaches. J Loss Prevent Proc Ind. 2012;25:142–7.
Omrani A, Simon LC, Rostami AA, Ghaemy M. Cure kinetics, dynamic mechanical and morphological properties of epoxy resin–Im6NiBr 2 system. Eur Polym J. 2008;44:769–79.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Tsai, YT., Cao, CR. et al. Kinetic and thermal safety analysis for tert-butyl peroxy-3,5,5-trimethylhexanoate by advanced calorimetric technology. J Therm Anal Calorim 127, 2253–2262 (2017). https://doi.org/10.1007/s10973-016-5778-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5778-y