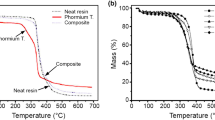
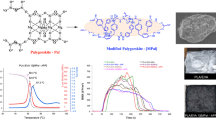
Abstract
(3-4-Epoxycyclohexane) methyl-3′-4′-epoxycyclohexyl-carboxylate (EEC) is a typical epoxy resin (EP). In Asia, due to the unstable reactive natures of EP, various thermal hazard and runaway reaction incidents have been occasioned by EP in the manufacturing process, such as fire, explosion, and toxic release, resulting in loss of life as well financial catastrophes and social outcries. Certain catalysis substances, H2SO4, acetic acid, or NaOH, may accelerate the reaction or curing rate for EP. However, an incompatible reaction with these chemical substances may induce a thermal hazard, causing a runaway excursion during the last stage. We employed thermogravimetry (TG) to obtain thermal stability parameters under non-isothermal conditions to evaluate the runaway reactions for EEC. The experimental data were compared with kinetics-based curve fitting to assess thermally hazardous phenomena by optimizing curve fitting on the kinetic parameters. The aim of this study was to estimate the incompatible hazards for EEC, provide thermal hazard information in order to determine the optimum operation conditions, and diminish the likelihood of fire and explosion accidents incurred by EP.









Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor (m3 mol−1 s−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- dα/dt :
-
Conversion rate (s−1)
- r :
-
Correlation coefficient, −1 to 1 (dimensionless)
- R :
-
Gas constant (J K−1 mol−1)
- R 2 :
-
Coefficient of determination, 0–1 (dimensionless)
- T :
-
Absolute temperature (K)
- T 0 :
-
Onset temperature (°C)
- T 01 :
-
Onset temperature under first decomposition stage (°C)
- T 02 :
-
Onset temperature under second decomposition stage (°C)
- T 03 :
-
Onset temperature under third decomposition stage (°C)
- T f :
-
Final temperature (°C)
- T p :
-
Peak temperature (°C)
- A(α):
-
Pre-exponential factor with degree of conversion (m3 mol−1 s−1)
- E(α):
-
Apparent activation energy with degree of conversion (kJ mol−1)
- t :
-
Time (s)
- α :
-
Degree of conversion (dimensionless)
- β :
-
Heating rate (°C min−1)
References
Chrissafis K, Roumeli E, Paraskevopoulos KM, Nianias N, Bikiaris DN. Effect of different nanoparticles on thermal decomposition of poly (propylene sebacate)/nanocomposites: evaluation of mechanisms using TGA and TG–FTIR–GC/MS. J Anal Appl Pyrolysis. 2012;96:92–9.
Yoo MJ, Kim SH, Park SD, Lee WS, Sun JW, Choi JH, Sahn N. Investigation of curing kinetics of various cycloaliphatic epoxy resins using dynamic thermal analysis. Eur Polym J. 2010;46:1158–62.
Park SJ, Jin FL. Thermal stabilities and dynamic mechanical properties of sulfone-containing epoxy resin cured with anhydride. Polym Degrad Stab. 2004;86:515–20.
Wu HC, Chang RC, Ou HJ, Peng DJ. Case study on prevention of fire hazards in coating epoxy-based FRP work with illumination. J Loss Prevent Proc Ind. 2010;23:346–50.
Marta W. The influence of tertiary aromatic amines on the BPO initiated cure of unsaturated epoxy polyesters with styrene studied by non-isothermal DSC. J Therm Anal Calorim. 2011;105:987–94.
Morita A. Cationic polymerization of hydrogenated bisphenol-A glycidyl ether with cycloaliphatic epoxy resin and its thermal discoloration. J Appl Polym Sci. 2005;97:1395–440.
Ochi M, Ichikawa N, Harada M, Hara M, Uchida H. Toughening of cycloaliphatic epoxy resin improved by reactive copolymers with flexible alkyl side chains. J Appl Polym Sci. 2012;124:4572–8.
Catalani A, Bonicelli MG. Kinetics of the curing reaction of a diglycidyl ether of bisphenol A with a modified polyamine. Thermochim Acta. 2005;438:126–9.
Omrani A, Simon LC, Rostami AA, Ghaemy M. Cure kinetics, dynamic mechanical and morphological properties of epoxy resin–Im6NiBr2 system. Eur Polym J. 2008;44:769–79.
Ramírez C, Rico M, Torres A, Barral L, López J, Montero M. Macromolecular nanotechnology-Review epoxy/POSS organic–inorganic hybrids: ATR-FTIR and DSC studies. Eur Polym J. 2008;44:3035–45.
Morita Y. Cationic polymerization of hydrogenated bisphenol-A glycidyl ether with cycloaliphatic epoxy resin and its thermal discoloration. J Appl Polym Sci. 2005;97:1395–400.
Sangermano M, Yagci Y, Rizza G. In situ synthesis of silver-epoxy nanocomposites. Macromolecules. 2007;40:8827–9.
Chitkara D, Kumar N. BSA-PLGA-based core-shell nanoparticles as carrier system for water-soluble drugs. Pharm Res-Dordr. 2013;30:2396–409.
Hou HY, Duh YS, Lin WH, Shu CM. Reactive incompatibility of cumene hydroperoxide mixed with alkaline solutions. J Therm Anal Calorim. 2006;85:145–50.
Tsai YT, You ML, Qian XM, Shu CM. Calorimetric techniques combined with various thermokinetic models to evaluate incompatible hazard of tert-butyl peroxy-2-ethyl hexanoate mixed with metal ions. Ind Eng Chem Res. 2013;52:8206–15.
Chu YC, Tsai FC, Lin FR, Chiang TC, Shu CM. Evaluation unexpected energy released for three liquid organic peroxides. Energy Educ Sci Tech Pt A. 2013;30:977–82.
Lu KM, Lee WJ, Chen WH, Lin TC. Thermogravimetric analysis and kinetics of co-pyrolysis of raw/torrefied wood and coal blends. Appl Energy. 2013;105:57–65.
Li AC, Tsai YT, Wu SH, Chiu CW, Shen SJ, Chang RH, Shu CM. Thermal runaway analyses for two organic peroxides with H2O and dry fire-extinguishing chemicals by DSC and VSP2. J Therm Anal Calorim. 2013;113:1611–8.
Wang TS, Liu SH, Qian XM, You ML, Chou WL, Shu CM. Isothermal hazards evaluation of benzoyl peroxide mixed with benzoic acid via TAM III test. J Therm Anal Calorim. 2013;113:1625–31.
Lin CP, Chang YM, Tseng JM, Shu CM. Comparisons of nth-order kinetic algorithms and kinetic model simulation on HMX by DSC tests. J Therm Anal Calorim. 2010;100:607–14.
Jeske H, Schirp A, Cornelius F. Development of a thermogravimetric analysis (TGA) method for quantitative analysis of wood flour and polypropylene in wood plastic composites (WPC). Thermochim Acta. 2012;543:165–71.
Chi JH, Wu SH, Shu CM. Thermal explosion analysis of methyl ethyl ketone peroxide by non-isothermal and isothermal calorimetric applications. J Hazard Mater. 2009;171:1145–9.
Wang YW, Liao MS, Shu CM. Thermal runaway hazards for LiCoO2 Li-ion batteries by DSC tests. Energy Educ Sci Tech Pt A. 2012;30:1–4.
Lee MH, Chen JR, Shiue GY, Lin YF, Shu CM. Simulation approach to benzoyl peroxide decomposition kinetics by thermal calorimetric technique. J Taiwan Inst Chem E. 2013. doi:10.1016/j.jtice.2013.06.002.
Zang Na, Qian XM, Liao JY, Shu CM. Thermal stability of lauroyl peroxide by isoconversional kinetics evaluation and finite element analysis. J Taiwan Inst Chem Eng. 2013. doi:10.1016/j.jtice.2013.06.004.
Weng SY, Liu SH, Tsai LC, Hsieh TF, Ma CM, Shu CM. Thermokinetics simulation for multi-walled carbon nanotubes with sodium alginate by advanced kinetics and technology solutions. J Therm Anal Calorim. 2013;113:1603–10.
Tehfe MA, Lalevee J, Gigmes D, Fouassier JP. Green chemistry: sunlight-induced cationic polymerizationof renewable epoxy monomers under air. Macromolecules. 2010;43:1364–70.
Alzina C, Mija A, Vincent L, Sbirrazzuoli N. Effects of incorporation of organically modified montmorillonite on the reaction mechanism of epoxy/amine cure. J Phys Chem B. 2012;116:5786–94.
Jin FL, Park SJ. Thermal properties of epoxy resin/filler hybrid composites. Polym Degrad Stab. 2012;97:2148–53.
Ahamad T, Alshehri SM. Thermal degradation and evolved gas analysis of epoxy (DGEBA)/novolac resin blends (ENB) during pyrolysis and combustion. J Therm Anal Calorim. 2013;111:445–51.
Rosu D, Rosu L, Brebu M. Thermal stability of silver sulfathiazole–epoxy resin network. J Anal Appl Pyrol. 2011;92:10–8.
Cai HY, Li P, Sui G, Yu YH, Li G, Yang XP, Ryu SK. Curing kinetics study of epoxy resin/flexible amine toughness systems by dynamic and isothermal DSC. Thermochim Acta. 2008;473:101–5.
Ramírez C, Rico M, Torres A, Barral L, López J, Montero B. Epoxy/POSS organic–inorganic hybrids: ATR-FTIR and DSC studies. Eur Polym J. 2008;44:3035–45.
Bacosca I, Hamciuc E, Cristea M, Lisa G, Bruma M. Poly(ether imide)s containing cyano substituents and thin films made from them. Thermochim Acta. 2012;124:1956–66.
Jhu CY, Wang YW, Wen CY, Shu CM. Thermal runaway potential of LiCoO2 and Li(Ni1/3Co1/3Mn1/3)O2 batteries determined with adiabatic calorimetry methodology. Appl Energy. 2012;100:127–31.
Liu SH, Hou HY, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids and alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;556:226–32.
Liu X, Yu L, Xie F, Li M, Chen L, Xiao Li. Kinetics and mechanism of thermal decomposition of cornstarches with different amylose/amylopectin ratios. Starch-Starke. 2010;62:139–46.
Adnadjevic B, Lazarevic N, Jovanovic J. A study of the kinetics of isothermal nicotine desorption from silicon dioxide. Appl Surf Sci. 2010;257:1425–30.
Alonso MV, Oliet M, Garc′ıa J, Rodr′ıguez F, Echeverr′ıa J. Gelation and isoconversional kinetic analysis of lignin–phenol–formaldehyde resol resins cure. Chem Eng J. 2006;122:159–66.
Khawam A, Flanagan DR. Role of isoconversional methods in varying activation energies of solid-state kinetics: I. Isothermal kinetic studies. Thermochim Acta. 2005;429:93–102.
Omrani A, Simon LC, Rostami AA, Ghaemy M. Cure kinetics, dynamic mechanical and morphological properties of epoxy resin–Im6NiBr 2 system. Eur Polym J. 2008;44:769–79.
Pérez JM, Oliet M, Alonso MV, Rodr′ıguez F. Cure kinetics of lignin–novolac resins studied by isoconversional methods. Thermochim Acta. 2009;487:39–42.
Acknowledgements
This study was supported by the National Science Council (NSC) of Taiwan under grant number NSC101-2221-E-407-001-MY3.
Author information
Authors and Affiliations
Corresponding author
Additional information
BW-17, Thermodynamics, Liquid Crystal, and Crystallization Session, Presented at NATAS 2013, August 4–7, 2013, Bowling Green, Kentucky, USA.
Rights and permissions
About this article
Cite this article
Tong, JW., Chen, WC., Tsai, YT. et al. Incompatible reaction for (3-4-epoxycyclohexane) methyl-3′-4′-epoxycyclohexyl-carboxylate (EEC) by calorimetric technology and theoretical kinetic model. J Therm Anal Calorim 116, 1445–1452 (2014). https://doi.org/10.1007/s10973-014-3685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3685-7