Abstract
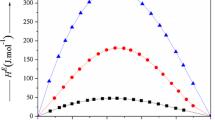
Molar heat of mixing and volume change of mixing, coinciding with molar excess enthalpies, H E, and molar excess volumes, V E, respectively, of binary mixtures composed by a linear saturated aliphatic acid of general formula: CH3(CH2)u-2–CO2H [u = 2, 3, 4, 5, 6, 7, 8] + di-iso-propyl ether, [(CH3)2CH−]2O, have been measured in the whole range of mole fraction at 298.15 K and 0.10 MPa. The H E experimental determinations have been realized by means of a mixing flow calorimetric apparatus, and V E have been calculated from density data acquired by using a vibrating tube densitometer. Mixing is exothermic for all investigated systems. The values at equimolar composition, H Ex=0.5 , are in the range: (−385 ± 1 to −140 ± 4) J mol−1. The more negative value is attributable to butanoic acid-containing mixture; then, H E decrease in absolute value as the alkyl chain of the acid increases. For the same systems, the V E trends are different: the value at equimolar composition, V Ex=0.5 , always negative, increase regularly in absolute value as the alkyl chain length of the acid increases from –1.12 × 10−6 m3 mol−1 for mixtures containing ethanoic acid to −1.75 × 10−6 m3 mol−1 for octanoic acid-containing mixtures.


Similar content being viewed by others
References
Riddick JA, Bunger WB, Sakano TK. Organic solvents. Physical properties and methods of purification. In: Weissberger A, editor. Techniques of chemistry, vol. 2. 4th ed. New York: Wiley; 1986.
Arivazhagan G, Shanmugam R, Thenappan T. Dielectric, FT–IR and UV–Vis spectroscopic studies on the fluid structure of diisopropyl ether–caprylic acid mixture. J Mol Struct. 2011;990:276–80.
Grolier JPE, Wormald CJ, Fontaine JC, Sosnkowska-Kehiaian K, Kehiaian HV. In: Kehiaian HV, editor. Heats of mixing and solutions, Binary Liquid Systems of Nonelectrolytes, Landolt-Börnstein - Group IV Physical Chemistry, Numerical data and functional relationships in science and technology, vol. 10A. Berlin: Springer; 2004.
Wilhelm E, Inglese A, Grolier J-PE. Excess molar enthalpies of (an alkanoic acid + n-heptane, or cyclohexane, or benzene) at T = 298.15 K. J Chem Thermodyn. 1999;31:1165–74.
Zhu S, Shen S, Benson GC, Lu BCY. Excess enthalpies of (2,4-dimethyl-3-oxapentane + one or more n-alkanes) at the temperature 298.15 K Part I: binary mixtures. J Chem Thermodyn. 1994;26(4):415–20.
Wang Z, Horikawa Y, Benson GC, Lu BCY. Excess enthalpies of the ternary mixtures: diisopropyl ether + n-octane + (n-heptane or n-dodecane) at 298.15 K. Fluid Phase Equilib. 2001;181:215–24.
Wang Z, Benson GC, Lu BCY. Excess enthalpies of the ternary mixtures: tetrahydrofuran + (hexane or cyclohexane) + decane at 298.15 K. J Chem Eng Data. 2003;48:190–4.
Brocos P, Calvo E, Bravo R, Pintos M, Amigo A, Roux A, Roux-Desgranges G. Heat capacities, excess enthalpies, and volumes of mixtures containing cyclic ethers. 3. Binary systems (tetrahydrofuran, tetrahydropyran, 1,4-dioxane or 1,3-dioxolane + cyclohexane or toluene). J Chem Eng Data. 1999;44:67–72.
Inglese A, Wilhelm E, Grolier JPE, Kehiaian HV. Thermodynamics of binary mixtures containing cyclic ethers. 2. Excess enthalpies of oxolane, 1,3-dioxolane, oxane, 1,3-dioxane and 1,4-dioxane with n-alkanes. J Chem Thermodyn. 1980;12:217–22.
Andrews AW, Morcom KW. Thermodynamic properties of some hydrocarbon + cyclic ethers mixtures. 2. Enthalpies of mixing. J Chem Thermodyn. 1971;3:519–25.
Wilhelm E, Inglese A, Grolier JPE, Kehiaian HV. Thermodynamics of binary mixtures containing cyclic ethers. 4. Excess enthalpies of oxolane + and oxane + alkanoic acid (HCO2H to C7H15CO2H). J Chem Thermodyn. 1982;14:33–6.
Marongiu B, Porcedda S, Falconieri D, Piras A, Matteoli L, Lepori L. Excess enthalpies of mixtures of mono-carboxylic acid with dibutylether. Comparison with DISQUAC predictions. J Thermal Anal Calorim. 2012;108:777–82.
Ulbig P, Geyer H, Gross O, Schulz S. Excess volumes for diisopropyl ether + butanoic acid and + butyl formate from 298.15 to 323.15 K at Pressures up to 60 MPa. J Chem Eng Data. 1998;43:175–7.
Bahadur I, Singh S, Deenadayalu N, Naidoo P, Ramjugernath D. Influence of alkyl group and temperature on thermophysical properties of carboxylic acid and their binary mixtures. Thermochim Acta. 2014;590:151–9.
Wieser ME, et al. Atomic weights of the elements 2011 (IUPAC Technical Report). Pure Appl Chem. 2013;85(5):1047–78.
Stokes RH, Levien BJ, Marsh KN. A continuous dilution dilatometer. The excess volume for the system cyclohexane + benzene. J Chem Thermodyn. 1970;2:43–52.
Marsh KN, Richards AE. Excess volumes of ethanol + water mixtures at 10-K intervals from 278.15 to 338.15 K. Aust J Chem. 1980;33:2121–32.
Sharma VK, Solanki S, Bhagour S. Excess molar enthalpies of binary and ternary liquid mixtures. J Thermal Anal Calorim. 2015;119:1293–302.
Monk P, Wadsö I. A flow micro reaction calorimeter. Acta Chem Scand. 1968;22:1842–52.
Marsh KN. Excess enthalpy of Benzene + Cyclohexane. Int Data Ser, Ser A. Sel Data Mixtures. 1973;1:1–5.
Redlch O, Kister AT. Thermodynamics of nonelectrolyte solutions. Ind Eng Chem. 1948;40(2):341–5.
Motin MA, Kabir MH, Huque EM. Densities and excess molar volumes of formic acid, acetic acid and propionic acid in pure water and in water + Surf Excel solutions at different temperatures. Phys Chem Liq. 2005;43(3):277–88.
Mato MM, Cebreiro SM, Legido JL, Andrade MIP. Study of the effect of increasing the chain length of the alkane in excess molar enthalpies of mixtures containing methyl tert-butyl ether. 1-propanol, alkane. Fluid Phase Equilib. 2008;27:6–12.
Li S, Yan W, Dong H. Determination of partial molar excess enthalpies at infinite dilution for the systems four alcohols + [bmim]PF6 at different temperatures by isothermal titration calorimeter. Fluid Phase Equilib. 2007;261:444–8.
Usula M, Plechkova NV, Piras A, Porcedda S. Ethylammonium alkanoate-based ionic liquid + water mixtures: a calorimetric and volumetric study at 298.15 K. J Thermal Anal Calorim. 2015;121:1129–37.
Arivazhagan G, Shanmugam R, Elangovan A. A probe on the intermolecular forces in diisopropyl ether–n–butyric acid mixture by dielectric, FTIR studies and quantum chemical calculations. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;105:102–8.
Acknowledgements
This research was financially supported by Fondazione Banco di Sardegna.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porcedda, S., Leo, M., Usula, M. et al. Excess enthalpies and excess volumes of binary mixtures containing a linear carboxylic acid + di-iso-propyl ether at 298.15 K and 0.1 MPa. J Therm Anal Calorim 125, 607–615 (2016). https://doi.org/10.1007/s10973-016-5504-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5504-9