Abstract
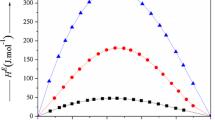
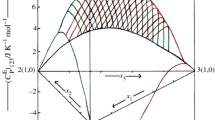
Excess molar enthalpies, \( H_{123}^{\text{E}} \) of ternary 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + 2-methyl aniline (2) + aniline or N-methyl aniline (3); 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + water (2) + formamide or N,N-dimethyl formamide (3) and H E of 2-methyl aniline (1) + aniline or N-methyl aniline (2); 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + formamide (2) mixtures, have been measured at 298.15 K. The excess molar enthalpies, \( H_{123}^{\text{E}} \), are negative for 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + 2-methyl aniline (2) + aniline or N-methyl aniline (3) mixtures and are positive for 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + water (2) + formamide (3) mixture over entire composition of (1) and (2) components. However, the magnitude and sign of \( H_{123}^{\text{E}} \) value for 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + water (2) + N,N-dimethyl formamide (3) mixture vary by the relative composition of the constituent molecules. The \( H_{123}^{\text{E}} \) and H E data have also been tested in terms of Graph and Prigogine–Flory–Patterson theories.





Similar content being viewed by others
References
Du C, Cai D, Qin M, Zheng P, Hao Z, Yin T, Zhao J, Shen W. Thermodynamics of mixed surfactant solutions of N,N-bis(dimethyldodecyl)-1,2-ethanediammonium dibromide with 1-dodecyl-3-methylimidazolium bromide. J Phys Chem B. 2014;118:1168–79.
Liu H, Yamashita T, Kamiyama T, Fujisawa M, Kimura T. Excess enthalpies of binary mixtures of ortho-, meta- and para-structural isomers. J Therm Anal Calorim. 2010;99:95–103.
Wang YH, Gao H, Yan WD. Excess molar enthalpies of diethyl malonate + (methanol, + ethanol, +1-propanol, and +2-propanol) at T = (288.2, 298.2, 313.2, and 328.2) K and p = 101.3 kPa. J Chem Eng Data. 2010;55:381–4.
Paduszyński K, Królikowski M, Domańska U. Excess enthalpies of mixing of piperidinium ionic liquids with short-chain alcohols: measurements and PC-SAFT modeling. J Phys Chem B. 2013;117:3884–91.
Sharma VK, Kataria J, Bhagour S. Thermodynamic investigations of 1-ethyl-3-methylimidazolium tetrafluoroborate and cycloalkanone mixtures. J Therm Anal Calorim. 2014;118:431–47.
Rebelo LPN, Najdanovic-Visak V, Visak ZP, Nunes da Ponte M, Szydlowski J, Cerdeiriña CA, Troncoso J, Romaní L, Esperança JMSS, Guedes HJR, de Sousa HCA. Detailed thermodynamic analysis of (C4mim)(BF4) + water as a case study to model ionic liquid aqueous solutions. Green Chem. 2004;6:369–81.
Sharma VK, Solanki S. Topological investigations of binary mixtures containing 1-ethyl-3-methyl imidazolium tetrafluoroborate and anilines. J Mol Liq. 2013;177:133–44.
Sharma VK, Solanki S, Bhagour S, Sharma D. Topological investigations of thermodynamic properties of ionic liquid mixtures: excess molar volumes and excess isentropic compressibilities. J Mol Liq. 2013;188:258–71.
Sharma VK, Bhagour S. Molecular interactions in 1-ethyl-3-methyl imidazolium tetrafluoroborate + amide mixtures: excess molar volumes and excess isentropic compressibilities and excess molar enthalpies. J Solut Chem. 2013;42:800–22.
Sharma VK, Bhagour S, Solanki S, Sharma D. Thermodynamic properties of ternary mixtures of 1-ethyl-3-methyl imidazolium tetrafluoroborate with 1-methyl pyrrolidin-2-one or pyrrolidin-2-one + water. Thermochim Acta. 2013;563:72–81.
Zhang X, Chang R, Zhao Z. Measurement and prediction of excess molar enthalpies of ternary working solutions 1-butyl-3-methylimidazolium dibutylphosphate + methanol/ethanol + water. Huagong Xuebao/CIESC J. 2013;64:1520–5.
Zang XD, Yu J. Measurement and correlation of excess mole enthalpy of ternary working solutions l-ethyl-3-methylimidazolium dimethylphosphate + methanol/or ethanol + water. J Eng Thermophys. 2013;34:1004–7.
Sharma VK, Solanki S, Bhagour S, Sharma D. Excess molar enthalpies of ternary mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate and organic solvents. Thermochim Acta. 2013;569:36–41.
Sharma VK, Bhagour S, Solanki S, Sheetal S, Jangra SK. Excess molar enthalpies for [emim][BF4] + pyrrolidin-2-one or 1-methylpyrrolidin-2-one + pyridine or water mixtures. J Chem Thermodyn. 2014;68:235–43.
Scholz E. Karl Fischer titration. Berlin: Springer-Verlag; 1984.
Riddick JA, Bunger WB, Sakano TK. Organic solvents physical properties and methods of purification. 4th ed. New York: Wiley; 1986.
Vogel AI. A text book of practical organic chemistry. 5th ed. London: English Book Society and Longman Group; 2003.
Saini N, Yadav JS, Jangra SK, Sharma D, Sharma VK. Thermodynamic studies of molecular interactions in mixtures of o-toulidine with pyridine and picolines: excess molar volumes, excess molar enthalpies, and excess isentropic compressibilities. J Chem Thermodyn. 2011;43:782–95.
Dubey GP, Sharma M. Temperature and composition dependence of the densities, viscosities, and speeds of sound of binary liquid mixtures of 1-butanol with hexadecane and squalane. J Chem Eng Data. 2008;53:1032–8.
Navia P, Troncoso J, Romani L. Excess magnitudes for ionic liquid binary mixtures with a common ion. J Chem Eng Data. 2007;52:1369–74.
Stoppa A, Zech O, Kunz W, Buchner R. The conductivity of imidazolium based ionic liquids from (−35 to 195) °C. A variation of cation’s alkyl chain. J Chem Eng Data. 2010;55:1768–73.
Neeti SK, Jangra SK, Yadav JS, Dimple N, Sharma VK. Thermodynamic investigations of ternary o-toluidine + tetrahydropyran + N,N-dimethyl formamide mixture and its binaries at 298.15, 303.15 and 308.15 K. J Mol Liq. 2011;163:36–45.
Alonso I, Mozo I, Fuente IGDL, González JA, Cobos JC. Thermodynamics of ketone + amine mixtures. Part III. Volumetric and speed of sound data at (293.15, 298.15, and 303.15) K for 2-butanone +aniline, +N-methylaniline, or +pyridine systems. J Chem Eng Data. 2010;55:5400–5.
Nakanishi K, Touhara H. Excess molar enthalpies of (methanol + aniline), (methanol + N-methylaniline), and (methanol + N,N-dimethylaniline). J Chem Thermodyn. 1986;18:657–60.
Sharma VK, Kumar S. Excess isentropic compressibilities for 1,3-dioxolane or 1,4-dioxane + water + formamide or N,N-dimethyl formamide ternary mixtures at 308.15 K. J Solut Chem. 2005;34:713–30.
García B, Alcalde R, Leal JM, Matos JS. Solute–solvent interactions in amide-water mixed solvents. J Phys Chem B. 1997;101:7991–7.
Papamatthaiakis D, Aroni F, Havredaki V. Isentropic compressibilities of (amide + water) mixtures: a comparative study. J Chem Thermodyn. 2008;40:107–18.
Murthy NM, Siva Kumar KV, Rajagopal E, Subrahmanyam SV. Excess thermodynamic and water-N,N-dimethylformamide. Acustica. 1981;48:341–5.
Sabbah R, Xu-wu An, Chickos JS, Leitão MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:137.
Singh PP, Sharma PK, Maken S. Topology and thermodynamic investigations of molecular interactions in some lower amides + water mixtures. Indian J Chem. 1992;31A:619–25.
Huggins ML. The thermodynamic properties of liquids included solutions: Part 1. Intermolecular energies in mono atomic liquids and their mixtures. J Phys Chem. 1970;74:371–8.
Huggins ML. The thermodynamic properties of liquids included solutions: Part 2. Polymer solutions considered as diatomic system. Polymer. 1971;12:389–99.
Singh PP, Bhatia M. Energetic of molecular interactions in binary mixtures of non-electrolytes containing a salt. J Chem Soc Faraday Trans I. 1989;85:3807–12.
Singh PP, Nigam RK, Singh KC, Sharma VK. Topological aspects of the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1981;46:175–90.
Yadav JS, Sharma D, Sharma VK. Topological investigations of thermodynamic properties of binary mixtures containing 2-pyrrolidinone. Thermochim Acta. 2009;489:45–52.
Sharma VK, Siwach RK, Dimple S. Excess molar volumes, excess molar enthalpies, and excess isentropic compressibilities of tetrahydropyran with aromatic hydrocarbons tetrahydropyran with aromatic hydrocarbons. J Chem Thermodyn. 2011;43:39–46.
Singh PP. Topological aspects of the effect of temperature and pressure on the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1983;66:37–73.
Kier LB, Yalkowasky SH, Sinkula AA, Valvan, SC. Physico-chemical properties of drugs (chap. 9). New York: Mercel Dekker; 1980, (a) p. 282, (b) p. 295.
Van HT, Patterson D. Volume of mixing and P* effect: Part 1. Hexane isomers with normal and branched hexadecane. J Solut Chem. 1982;11:793–805.
Flory PJ. The statistical thermodynamic of liquid mixtures. J Am Chem Soc. 1965;87:1833–8.
Flory PJ. The thermodynamic properties of mixture of small non-polar molecules. J Am Chem Soc. 1965;87:1838–46.
Sharma VK, Dua R. Topological and thermodynamic investigations of mixtures containing o-chlorotoluene and lower amides. J Chem Thermodyn. 2014;71:182–95.
Hildebrand JH, Prausnitz JM, Scot RL. Regular and related solutions: the solubility of gases, liquids, and solids. New York: Van Nonstand Reinhelds; 1971.
Acknowledgements
Subhash Solanki is grateful to CSIR, New Delhi, India for the award of SRF. The authors are also grateful to the Head of Chemistry Department and authorities of M. D. University, Rohtak, for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, V.K., Solanki, S. & Bhagour, S. Excess molar enthalpies of binary and ternary liquid mixtures. J Therm Anal Calorim 119, 1293–1302 (2015). https://doi.org/10.1007/s10973-014-4286-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4286-1