Abstract
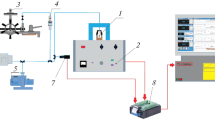
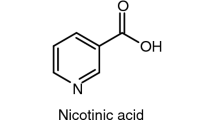
The inert gas transpiration technique was used for measuring the vapor pressures of the following biologically active compounds: 4-aminobenzoic acid, nicotinic acid, 4-aminobenzamide, 4-pyridinecarboxamide, N-(2-chloro-3-pyridinyl)-benzenesulfonamide, 4-nitro-N-2-thiazolyl-benzenesulfonamide, and 4-amino-N-(5-methyl-3-isoxazolyl)-benzenesulfon-amide in the appropriate temperature ranges. Differential scanning calorimetric analysis was applied to obtain the temperature and molar enthalpy of melting of the compounds studied. Standard molar enthalpies, entropies, and Gibbs energies of sublimation were derived at T = 298.15 K from the temperature dependences of the vapor pressure of the compounds. The obtained results allowed the estimation of the relationship between the sublimation thermodynamic parameters and the thermophysical properties of the substances. Correlation equations were used for calculating the saturated vapor pressures on the basis of the melting temperatures, sublimation enthalpies, and physicochemical HYBOT descriptors.



Similar content being viewed by others
References
Aromatic Hydrocarbons—Advances in Research and Treatment: 2013 Edition. Acton, A. Ed., ScholarlyEditions™: Atlanta, 2013
Akberova SI. Para-aminobenzoic acid and perspectives of its application in ophthalmology. Vestnic Ophthalmol. 2002;3:53–6.
Gracin S, Rasmuson ÅC. Polymorphism and crystallization of p-aminobenzoic acid. Cryst Growth Des. 2004;4:1013–23.
Olsen RA, Liu L, Ghaderi N, Johns A, Hatcher ME, Mueller LJ. The amide rotational barriers in picolinamide and nicotinamide: NMR and Ab initio studies. J Am Chem Soc. 2003;125:10125–32.
Bruckert E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210:353–61.
Maren TH. Relations between structure and biological activity of sulfonamides. Ann Rev Pharmacol Toxicol. 1976;16:309–27.
Owa T, Nagasu T. Novel sulphonamide derivatives for the treatement of cancer. Expert Opin Ther Pat. 2000;10:1725–40.
Winum J-Y, Scozzafava A, Montero J-L, Supuran CT. Sulfamates and their therapeutic potential. Med Res Rev. 2005;25:186–228.
De Kruif CG, Voogd J, Offringa JCA. Enthalpies of sublimation and vapour pressures of 14 amino acids and peptides. J Chem Thermodyn. 1979;11:651–6.
Domalski ES, Hearing ED. Estimation of the thermodynamic properties of C–H–N–O–S-Halogen compounds at 298.15 K. J Phys Chem Ref Data. 1993;22:805–55.
Rotich K, Glass BD, Brown ME. Thermal studies on some substituted aminobenzoic acids. J Therm Anal Calorim. 2001;64:681–8.
Almeida ARRP, Monte MJS, Agostinha M, Matos R, Morais VMF. Experimental and computational thermodynamic study of ortho- meta- and para-aminobenzamide. J Chem Thermodyn. 2013;59:222–32.
Ribeiro da Silva MDMC, Goncalves JM, Ferreira SCC, da Silva LCM, Sottomayor MJG. Experimental thermochemical study of the enthalpies of formation and sublimation of isonicotinamide, picolinamide, nicotinamide, isonicotinamide N-oxide, and nicotinamide N-oxide. The dissociation enthalpies of the N–O bonds. J Chem Thermodyn. 2001;33:1263–75.
Gonçalves EM, Bernardes CES, Diogo HP, Minas da Piedade ME. Energetics and structure of nicotinic acid (niacin). J Phys Chem B. 2010;114:5475–85.
Sabbah R, Chastel R, Laffitte M. Thermodynamics of nitrogen compounds. I. Calorimetric study of the enthalpies of sublimation of three aminobenzoic acids. Can J Chem. 1974;52:2201–5.
Aakeroy CB, Beatty AM, Helfrich BA. Do polymorphic compounds make good cocrystallizing agents? A structural case study that demonstrates the importance of synthon flexibility. Cryst Growth Des. 2003;3:159–65.
Lemmerer A, Bathori NB, Bourne SA. Chiral carboxylic acids and their effects on melting-point behaviour in co-crystals with isonicotinamide. Acta Crystallogr Sect B. 2008;64:780–90.
Vishweshwar P, Nangia A, Lynch VM. Molecular complexes of homologous alkanedicarboxylic acids with isonicotinamide: X-ray crystal structures, hydrogen bond synthons, and melting point alternation. Cryst Growth Des. 2003;3:783–90.
Fabian L, Hamill N, Eccles KS, Moynihan HA, Maguire AR, McCausland L, Lawrence SE. Cocrystals of fenamic acids with nicotinamide. Cryst Growth Des. 2011;11:3522–8.
Perlovich GL, Volkova TV, Manin AN, Bauer-Brandl A. Extent and mechanism of solvation and partitioning of isomers of substituted benzoic acids: a thermodynamic study in the solid state and in solution. J Pharm Sci. 2008;97:3883–96.
Manin AN, Voronin AP, Perlovich GL. Acetamidobenzoic acid isomers: studying sublimation and fusion processes and their relation with crystal. Thermochim Acta. 2014;583:72–7.
Ol’khovich MV, Blokhina SV, Sharapova AV, Perlovich GL, Proshin AN. Thermodynamics of sublimation and solvation for bicyclo-derivatives of 1,3-thiazine. Thermochim Acta. 2013;569:61–5.
Perlovich GL, Ryzhakov AM, Tkachev VV, Hansen LK, Raewsky OA. Sulfonamide molecular crystals: structure, sublimation thermodynamic characteristics, molecular packing, hydrogen bonds networks. Cryst Growth Des. 2013;13:4002–16.
Perlovich GL, Tkachev VV, Strakhova NN, Kazachenko VP, Volkova TV, Surov OV, Schaper KJ, Raevsky OA. Thermodynamic and structural aspects of sulfonamide crystals and solutions. J Pharm Sci. 2009;98:4738–55.
Perlovich GL, Strakhova NN, Kazachenko VP, Volkova TV, Tkachev VV, Schaper KJ, Raevsky OA. Studying thermodynamic aspects of sublimation, solubility and solvation processes and crystal structure analysis of some sulfonamides. Int J Pharm. 2007;334:115–24.
Perlovich GL, Strakhova NN, Kazachenko VP, Volkova TV, Tkachev VV, Schaper KJ, Raevsky OA. Sulfonamides as a subject to study molecular interactions in crystals and solutions: sublimation, solubility, solvation, distribution and crystal structure. Int J Pharm. 2008;349:300–13.
Chickos JS, Hosseini S, Hesse D, Liebman JF. Heat capacity corrections to a standard state: a comparison of new and some literature methods for organic liquids and solids. Struct Chem. 1993;4:271–8.
Zielenkiewicz W, Perlovich G, Wszelaka-Rylik M. The vapour pressure and the enthalpy of sublimation. Determination by inert gas flow method. J Therm Anal Calorim. 1999;57:225–34.
Ribeiro da Silva MAV, Monte MJS, Santos LMNB. J Chem Thermodyn. 2006;38:778–87.
Cox JD, Pilcher G. Thermochemistry of organic and organometallic compounds. London: Academic Press; 1970.
Chickos JS, Acree WE. Enthalpies of sublimation of organic and organometallic compounds. J Phys Chem Ref Data. 2002;2:537–698.
Raevsky OA, Grigor’ev VJ, Trepalin SV. HYBOT program package, Registration by Russian State Patent Agency No. 990090 of 26.02.99.
Eccles KS, Elcoate CJ, Maguire AR, Lawrence SE. Unzipping the dimer in primary amides by cocrystallization with sulfoxides. Cryst Growth Des. 2011;11:4433–9.
Habgood M, Deij MA, Mazurek J, Price SL, Horst JH. Carbamazepine co-crystallization with pyridine carboxamides: rationalization by complementary phase diagrams and crystal energy landscapes. Cryst Growth Des. 2010;10:903–12.
Joseph A, Bernardes CES, Minas da Piedade ME. Heat capacity and thermodynamics of solid and liquid pyridine-3-carboxylic acid (nicotinic acid) over the temperature range 296 to 531 K. J Chem Thermodyn. 2012;55:23–8.
Perlovich GL, Kazachenko VP, Strakhova NN, Raevsky OA. Impact of sulfonamide structure on solubility and transfer processes in biologically relevant solvents. J Chem Eng Data. 2014;59:4217–26.
Monte MJS, Santos LMNBF, Fonseca JMS, Sousa CAD. Vapor pressures, enthalpies and entropies of sublimation of para substituted benzoic acids. J Therm Anal Calorim. 2010;100:465–74.
Acree W, Chickos JS. Phase transition enthalpy measurements of organic and organometallic compounds. Sublimation, vaporization and fusion enthalpies from,1880 to 2010. J Phys Chem Ref Data. 2010;39(2010):1–942.
Harutada N, Solubility phenomena of pyridine- and pyrazinemonocarboxamides. I. Heat of sublimation of picolinamide, nicotinamide, isonicotinamide, and pyrazinecarboxamide. Takamine Kenkyusho Nempo. 1959; 11:66–75
Ribeiro da Silva MDMC, Goncalves JM, Acree WE Jr. Standard molar enthalpy of sublimation of crystalline 3-pyridinecarboxylic acid. J Chem Thermodyn. 2000;32:1071–3.
Sabbah R, Ider S. Thermodynamics of intermolecular and intramolecular bonds in three carboxypyridinic acids (picolinic, nicotinic and isonicotinic acids). Can J Chem. 1999;77:249–57.
Wright WB, King GSD. The crystal structure of nicotinic acid. Acta Cryst. 1953;6:305–17.
Blokhina SV, Sharapova AV, Ol’khovich MV, Volkova TV, Perlovich GL. Vapor pressures and thermodynamic sublimation of antitubercular drugs pyrazinamide and hydrazides isonicotinic acid. J Therm Anal Calorim. 2015;120:1053–60.
Perlovich GL, Ryzhakov AM, Tkachev VV, Hansen LK. Sulfonamide molecular crystals: thermodynamic and structural aspects. Cryst Growth Des. 2011;11:1067–81.
Perlovich GL, Raevsky OA. Sublimation of molecular crystals: Prediction of sublimation functions on the basis of HYBOT physicochemical descriptors and structural clusterization. Cryst Growth Des. 2010;10:2707–12.
Acknowledgements
This work was supported by the grant of Russian Science Foundation No. 14-13-00640. We thank “the Upper Volga Region Centre of Physicochemical Research” for technical assistance with DSC experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Volkova, T.V., Blokhina, S.V., Ryzhakov, A.M. et al. Vapor pressure and sublimation thermodynamics of aminobenzoic acid, nicotinic acid, and related amido-derivatives. J Therm Anal Calorim 123, 841–849 (2016). https://doi.org/10.1007/s10973-015-4969-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4969-2