Abstract
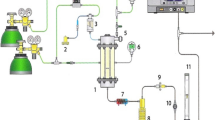
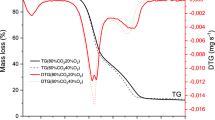
The aim of this work was on the thermal characterization of wastes of the sugarcane industry, such as bagasse, filter cake and vinasse, in both forms: pure and blended. Thermogravimetric analysis (TG), derivative thermogravimetric (DTG) and differential thermal analysis (DTA) were used for the evaluation of the thermal behavior of the samples under four different atmospheres: N2, CO2, N2/O2 and CO2/O2. Comparison of thermal behavior of the samples under combustion (N2/O2) and oxy-combustion (CO2/O2) reveals that replacing N2 by CO2 causes displacement of mass loss steps to higher temperatures and increases some DTG peaks. Higher heat capacity of carbon dioxide and higher partial pressure of CO2 molecules in relation to N2 ones explain these observations. Under CO2 (100 %) environment, an endothermic event—due to CO release—is observed at around 900 °C, which is attributed to the reverse Boudouard reaction. Interestingly, in all samples, when vinasse is present, such endothermic event starts at lower temperature (~700 °C), which can be understood as a reaction catalyzed by the high potassium content in the vinasse. Synergistic effect studies indicated that bagasse improved reactivity of blends due to its higher volatile content. Since there are no reports regarding the thermal characterization of wastes of the sugarcane industry under combustion, oxy-combustion and gasification atmospheres, this work establishes an important database for the study of similar types of biomass in the field of bioenergy.










Similar content being viewed by others
References
Adhikari DK, Seal D, Saxena C, Goyal HB. Biomass based fuel/energy. J Petrotech Soc. 2006;3(1):28–42.
Goyal HB, Saxena C, Seal D. Thermochemical conversion of biomass to liquids and gaseous fuels. In: Pandey A, editor. Handbook of plant-based biofuels. Boca Raton: CRC Books; 2009. p. 30–1.
Chen G, Andries J, Spliethoff H. Catalytic pyrolysis of biomass for hydrogen rich fuel gas production. Energy Convers Manage. 2003;44:2289–96.
Hansen J, Ruedy R, Glascoe J, Sato M. GISS analysis of surface temperature change. J Geophys Res. 1999;104:30997–1002.
Wall T, Liu Y, Spero C, Elliott L, Khare S, Rathnam R, Zeenathal F, Moghtaderi B, Buhre B, Sheng C, Gupta R, Yamada T, Makino K, Yu J. An overview on oxyfuel coal combustion—state of the art research and technology development. Chem Eng Res Des. 2009;87:1003–16.
Arias B, Pevida C, Rubiera F, Pis JJ. Effect of biomass blending on coal ignition and burnout during oxy-fuel combustion. Fuel. 2008;87:2753–9.
Toftegaard MB, Brix J, Jensen PA, Glarborg P, Jensen AD. Oxy-fuel combustion of solid fuels. Prog Energy Combust Sci. 2010;36:581–625.
Barnes AC. The sugar cane. London: Leonard Hill; 1964. p. 365–6.
Mutton MA, Rossetto R, Mutton MJR. Utilização agrícola da vinhaça. In: Cortez LAB, coordinator. Bioetanol de cana-de-açúcar: P&D para produtividade e sustentabilidade. São Paulo: Blücher; 2010, p. 423–40.
Ramalho JF, Amaral Sobrinho NM. Metais pesados em solos cultivados com cana-de-açúcar pelo uso de resíduos agroindustriais. Revista Floresta e Ambiente. 2001;8(1):120–9.
Agarwal CS, Pandey GS. Soil pollution by spent wash discharge: depletion of manganese (II) and impairment of its oxidation. J Environ Biol. 1994;15:49–53.
Silva MAS, Griebeler NP, Borges LC. Uso da vinhaça e impactos nas propriedades do solo e lençol freático. Revista Brasileira de Engenharia Agrícola e Ambiental. 2007;11(1):108–14.
Demirbas A. Fuels from biomass. In: Demirbas A, editor. Biorefineries: for biomass upgrading facilities. London: Springer; 2010. p. 33–73.
Cortez LAB, Silva A, Lucas J Jr, Jordan RA, Castro LR. Biodigestão de efluentes. In: Cortez LAB, Lora ES, editors. Biomassa para energia. Campinas: Editora da UNICAMP; 2007. p. 493–529.
González JF, García CMG, Ramiro A, González J, Sabio E, Gañán J, Rodríguez MA. Combustion optimisation of biomass residue pellets for domestic heating with a mural boiler. Biomass Bioenerg. 2004;27(2):145–54.
Öhman M, Boman C, Hedman H, Nordin A, Bostrom D. Slagging tendencies of wood pellet ash during combustion in residential pellet burners. Biomass Bioenergy. 2004;27(6):585–96.
Nussbaumer T. Combustion and co-combustion of biomass: fundamentals, technologies and primary measures for emission reduction. Energy Fuels. 2003;17:1510–21.
Nielsen HP, Frandsen FJ, Dam-Johansen K, Baxter LL. The implications of chlorine-associated corrosion on the operation of biomass-fired boilers. Prog Energy Combust Sci. 2000;26:283–98.
Backman R, Khalil RA, Todorovic D, Skreiberg O, Becidan M, Goile F, Skreiberg A, Sorum L. The effect of peat ash addition to demolition wood on the formation of alkali, lead and zinc compounds at staged combustion conditions. Fuel Process Technol. 2013;105:20–7.
Demirbas A. Sustainable cofiring of biomass with coal. Energy Convers Manag. 2003;44:1465–79.
Biagini E, Lippi F, Petarca L, Tognotti L. Devolatilization rate of biomasses and coal-biomass blends: an experimental investigation. Fuel. 2002;81:1041–50.
Baxter L. Biomass-coal co-combustion: opportunity for affordable renewable energy. Fuel. 2005;84:1295–302.
Wigmans ST, Göebel JC, Moulijn JA. The influence of pretreatment conditions on the activity and stability of sodium and potassium catalysts in carbon–steam reactions. Carbon. 1983;21(3):291–301.
Veraa MJ, Bell AT. Effect of alkali metal catalysts on gasification of coal char. Fuel. 1978;57:194–200.
Yokoyama S, Miyahara K, Tanaka K, Takakuwa I, Tashiro J. Catalytic reduction of carbon dioxide. 1. Reduction of carbon dioxide with carbon carrying potassium carbonate. Fuel. 1979;58:510–4.
Mckee DW, Spiro CL, Kosky PG, Lamby EJ. Catalysis of coal char gasification by alkali metal salts. Fuel. 1983;62(2):217–20.
Krerkkaiwan S, Fushimi C, Tsutsumi A, Kuchontara P. Synergetic effect during co-pyrolysis/gasification of biomass and sub-bituminous coal. Fuel Process Technol. 2013;115:11–8.
Lahijani P, Zainal ZA, Mohamed AR, Mohammadi M. CO2 gasification reactivity of biomass char: catalytic influence of alkali, alkaline earth and transition metal salts. Bioresour Technol. 2013;144:288–95.
Nemanova V, Abedini A, Liliedahl T, Engvall K. Co-gasification of petroleum coke and biomass. Fuel. 2014;117:870–5.
Gil MV, Oulego P, Casal MD, Pevida C, Pis JJ, Rubiera F. Mechanical durability and combustion characteristics of pellets from biomass blends. Bioresour Technol. 2010;101:8859–67.
Lajiji M, Limousy L, Jeguirim M. Physico-chemical properties and thermal degradation characteristics of agropellets from olive mill by-products/sawdust blends. Fuel Process Technol. 2014;1126:215–21.
Jirícek I, Rudasová P, Zemlová T. A thermogravimetric study of the behavior of biomass blends during combustion. Acta Polytech. 2012;52(3):39–42.
Jones JM, Darvell LI, Bridgeman TG, Pourkashanian M, Williams A. An investigation of thermal and catalytic behaviour of potassium in biomass combustion. Proc Combust Inst. 2007;31:1955–63.
Jesus HC, Costa EA, Mendonça ASF, Zandonade E. Distribuição de metais pesados em sedimentos do sistema estuarino da ilha de Vitória-ES. Quim Nova. 2004;27(3):376–86.
Kastanaki E, Vamvuka D, Grammelis P, Kakaras E. Thermogravimetric studies of the behavior of lignite–biomass blends during devolatilization. Fuel Process Technol. 2002;77:159–66.
Glória NA. Utilização agrícola da vinhaça. Brasil Açucareiro. 1975;86:11–7.
Bhosale PR, Chonde SG, Nakade DB, Raut PD. Studies on physic-chemical characteristics of waxed and dewaxed pressmud and its effect on water holding capacity of soil. ISCA J Biol Sci. 2012;1(1):35–41.
Carlson CL, Adriano DC. Environmental impacts of coal combustion residues. J Environ Qual. 1993;22:227–47.
Kumar R, Mago G, Balan V, Wyman CE. Physical and chemical characterization of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour Technol. 2009;100:3948–62.
Chen H, Liu N, Fan W. Two-step consecutive reaction model of biomass thermal decomposition by DSC. Acta Physicochim Sin. 2006;22(7):786–90.
Sutton D, Kelleher B, Ross JRH. Review of literature on catalysts for biomass gasification. Fuel Process Technol. 2001;73:155–73.
Vamvuka D, Karouki E, Sfakiotakis S, Salatino P. Gasification of waste biomass chars by carbon dioxide via thermogravimetry—effect of catalysts. Combust Sci Technol. 2012;184(1):64–77.
Mckee DW, Chatterji D. The catalytic effect of alkali metal carbonates and oxides in graphite oxidation reactions. Carbon. 1975;13:381–90.
Kopyscinski J, Rahman M, Gupta R, Mims CA, Hill JM. K2CO3 catalysed CO2 gasification of ash-free coal. Interactions of the catalyst with carbon in N2 and CO2 atmosphere. Fuel. 2014;117:1181–9.
Edreis EMA, Luo G, Li A, Chao C, Hu H, Zhang S, Gui B, Xiao L, Xu K, Zhang P, Yao H. CO2 co-gasification of lower sulphur petroleum coke and sugar cane bagasse via TG-FTIR analysis technique. Bioresour Technol. 2013;136:595–603.
Haas J, Tamura M, Weber R. Characterization of coal blends for pulverised fuel combustion. Fuel. 2001;80:1317–23.
Acknowledgements
The authors are grateful to the Institute of Chemistry (IQ/CAr) of UNESP and University of São Paulo (USP). We would like to thank the sugarcane mills for providing the samples used in this work. The main author would like to acknowledge CAPES for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, D.R., Crespi, M.S., Crnkovic, P.C.G.M. et al. Pyrolysis, combustion and oxy-combustion studies of sugarcane industry wastes and its blends. J Therm Anal Calorim 121, 309–318 (2015). https://doi.org/10.1007/s10973-015-4532-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4532-1