Abstract
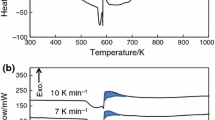
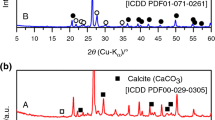
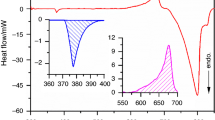
In this study, the kinetic behavior of the sodium (Na)–silica (SiO2) reaction was investigated for an assessment method of reactivity/stability of siliceous concrete against the sodium–concrete reaction (SCR) by postulating a severe accidental condition in the sodium-cooled fast reactor (SFR). The reaction behavior was tracked using a differential scanning calorimetry (DSC) equipped with a videoscope for viewing the changes in the sample during the reaction. The reaction was characterized as a partially overlapping multistep process controlled by physico-geometrical reaction scheme. The kinetic behavior in view of the change in the temperature at the maximum reaction rate with heating rate was analyzed using the simplified Kissinger method and the approximated Ozawa method. Further by separating the kinetic data for the overall reaction into five component reaction stages, the kinetic information for the second and third reaction stages was extracted. By comparing the kinetic results, it was revealed that the kinetic results determined from the kinetic data at the maximum reaction rate can be interpreted as is for the major reaction stage (the third reaction stage) controlled by an autocatalytic reaction. In addition, the second reaction stage was characterized as a possible premonitory process for the major reaction stage. Through the comprehensive interpretation of the kinetic results, significance of the kinetic analysis for the Na–SiO2 reaction using DSC for the reactivity/stability evaluation against SCR and for the safety assessment of SFR is discussed.











Similar content being viewed by others
References
Casselman C. Consequences of interaction between sodium and concrete. Nucl Eng Des. 1982;68(2):207–12. doi:10.1016/0029-5493(82)90032-2.
Chasanov MG, Staahl GE. High-temperature sodium–concrete interactions. J Nucl Mat. 1977;66(1–2):217–20. doi:10.1016/0022-3115(77)90154-4.
Suo-Anttila AJ. SLAM—A sodium-limestone concrete ablation model. Nuclear Regulatory Commission Contractor Report. 1983; NUREG/CR-3379 (SAND83-7114 R7).
Das SK, Sharma AK, Parida FC, Kasinathan N. Experimental study on thermo-chemical phenomena during interaction of limestone concrete with liquid sodium under inert atmosphere. Const Build Mat. 2009;23(11):3375–81. doi:10.1016/j.conbuildmat.2009.06.021.
Mohammed Haneefa K, Santhanam M, Parida FC. Review of concrete performance at elevated temperature and hot sodium exposure applications in nuclear industry. Nucl Eng Des. 2013;258:76–88. doi:10.1016/j.nucengdes.2013.01.018.
Unemoto T, Hashimoto Y, Takasaki S, Arakawa T, Yokoo J, Sasagawa Y. Experimental studies on sodium–concrete reaction (II). PNC Technical Report. 1983; PNC-TJ270 83-01.
Miyahara S, Haga K, Himeno Y. Sodium aerosol release rate and nonvolatile fission-product retention factor during a sodium–concrete reaction. Nucl Technol. 1992;97(2):212–26.
Chawla TC, Pedersen DR. A review of modeling concepts for sodium concrete reactions and a model for liquid-sodium transport to the unreacted concrete surface. Nucl Eng Des. 1985;88(1):85–91. doi:10.1016/0029-5493(85)90047-0.
Zhang B, Zhu J, Shan JXW. Simulation of chemical kinetics in sodium–concrete interactions. Nucl Sci Tech. 2006;17(1):53–60. doi:10.1016/s1001-8042(06)60012-2.
Bae JH, Shin MS, Min BH, Kim SM. Experimental study on sodium–concrete reactions. J Korean Nucl Soc. 1998;30(6):568–80.
Glasser LSD, Kataoka N. The chemistry of alkali-aggregate reaction. Cement Concr Res. 1981;11(1):1–9.
Davies G, Oberholster RE. Alkali-silica reaction-products and their development. Cement Concr Res. 1988;18(4):621–35. doi:10.1016/0008-8846(88)90055-5.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6. doi:10.1021/ac60131a045.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6. doi:10.1246/bcsj.38.1881.
Koga N, Goshi Y, Yamada S, Pérez-Maqueda LA. Kinetic approach to partially overlapped thermal decomposition processes. J Therm Anal Calorim. 2013;111(2):1463–74. doi:10.1007/s10973-012-2500-6.
Kikuchi S, Seino H, Kurihara A, Ohshima H. Kinetic study of sodium–water surface reaction by differential thermal analysis. J Pow Energy Syst. 2013;7(2):79–93. doi:10.1299/jpes.7.79.
Chase MWJ. JANAF thermochemical tables third edition part II, Cr–Zr. Amer Inst Phys.; 1986.
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Hack K, et al. FactSage thermochemical software and databases—recent developments. Calphad. 2009;33(2):295–311. doi:10.1016/j.calphad.2008.09.009.
Ukai S, Yoshida E, Enokida Y, Nihei I. Quantitative characterization on dissolution and deposition behavior of SUS316 stainless steel cladding constituents under flowing sodium in fast reactor. International conference on materials for nuclear reactor core applications; 1987; London (UK): British Nuclear Energy Society.
Bin Jenn S, Wu PCS, Chiotti P. Thermodynamic properties of the double oxides of Na2O with the oxides of Cr, Ni and Fe. J Nucl Mat. 1977;67(1–2):13–23. doi:10.1016/0022-3115(77)90157-x.
Koga N, Šesták J, Simon P. Some fundamental and historical aspects of phenomenological kinetics in the solid state studied by thermal analysis. In: Šesták J, Simon P, editors. Thermal analysis of micro, nano- and non-crystalline materials. Springer: Berlin; 2013. p. 1–28.
Koga N. Ozawa’s kinetic method for analyzing thermoanalytical curves: history and theoretical fundamentals. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-012-2882-5.
Westrich HR, Stockman HW, Suo-Anttila A. Laboratory-scale sodium-carbonate aggregate concrete interactions. Nuclear Regulatory Commission Contractor Report. 1983;NUREG/CR-3401: SAND83-1502 R7.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92.
Koga N, Šesták J. Further aspects of the kinetic compensation effect. J Therm Anal. 1991;37(5):1103–8. doi:10.1007/bf01932804.
Koga N, Šesták J. Kinetic compensation effect as a mathematical consequence of the exponential rate-constant. Thermochim Acta. 1991;182(2):201–8. doi:10.1016/0040-6031(91)80005-4.
Koga N. A review of the mutual dependence of Arrhenius parameters evaluated by the thermoanalytical study of solid-state reactions: the kinetic compensation effect. Thermochim Acta. 1994;244(1):1–20. doi:10.1016/0040-6031(94)80202-5.
Galwey AK, Mortimer M. Compensation effects and compensation defects in kinetic and mechanistic interpretations of heterogeneous chemical reactions. Int J Chem Kinet. 2006;38(7):464–73. doi:10.1002/kin.20176.
Koga N, Tanaka H. A physico-geometric approach to the kinetics of solid-state reactions as exemplified by the thermal dehydration and decomposition of inorganic solids. Thermochim Acta. 2002;388(1–2):41–61. doi:10.1016/s0040-6031(02)00051-5.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, et al. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23. doi:10.1016/j.tca.2014.05.036.
Friedman HL. Kinetics of thermal degradation of cha-forming plastics from thermogravimetry, application to a phenolic plastic. J Polym Sci C. 1964;6:183–95.
Sánchez-Jiménez PE, Perejón A, Criado JM, Diánez MJ, Pérez-Maqueda LA. Kinetic model for thermal dehydrochlorination of poly(vinyl chloride). Polymer. 2010;51(17):3998–4007. doi:10.1016/j.polymer.2010.06.020.
Yoshikawa M, Yamada S, Koga N. Phenomenological interpretation of the multistep thermal decomposition of silver carbonate to form silver metal. J Phys Chem C. 2014;118(15):8059–70. doi:10.1021/jp501407p.
Noda Y, Koga N. Phenomenological kinetics of the carbonation reaction of lithium hydroxide monohydrate: Role of surface product layer and possible existence of a liquid phase. J Phys Chem C. 2014;118(10):5424–36. doi:10.1021/jp500322p.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12. doi:10.1016/0040-6031(71)85051-7.
Šesták J. Diagnostic limits of phenomenological kinetic models introducing the accommodation function. J Therm Anal. 1990;36(6):1997–2007. doi:10.1007/bf01914116.
Šesták J. Rationale and fallacy of thermoanalytical kinetic patterns. J Therm Anal Calorim. 2011;110(1):5–16. doi:10.1007/s10973-011-2089-1.
Perez-Maqueda LA, Criado JM, Sánchez-Jiménez PE. Combined kinetic analysis of solid-state reactions: a powerful tool for the simultaneous determination of kinetic parameters and the kinetic model without previous assumptions on the reaction mechanism. J Phys Chem A. 2006;110(45):12456–62. doi:10.1021/jp064792g.
Koga N, Suzuki Y, Tatsuoka T. Thermal dehydration of magnesium acetate tetrahydrate: formation and in situ crystallization of anhydrous glass. J Phys Chem B. 2012;116(49):14477–86. doi:10.1021/jp3052517.
Málek J, Criado JM. Empirical kinetic models in thermal analysis. Thermochim Acta. 1992;203:25–30. doi:10.1016/0040-6031(92)85182-u.
Wada T, Koga N. Kinetics and mechanism of the thermal decomposition of sodium percarbonate: role of the surface product layer. J Phys Chem A. 2013;117(9):1880–9. doi:10.1021/jp3123924.
Kitabayashi S, Koga N. Physico-geometrical mechanism and overall kinetics of thermally induced oxidative decomposition of tin(ii) oxalate in air: formation process of microstructural tin(iv) oxide. J Phys Chem C. 2014;118(31):17847–61. doi:10.1021/jp505937k.
Svoboda R, Cicmanec P, Málek J. Kissinger equation versus glass transition phenomenology. J Therm Anal Calorim. 2013;114(1):285–93. doi:10.1007/s10973-012-2892-3.
Koga N, Šesták J, Málek J. Distortion of the Arrhenius parameters by the inappropriate kinetic-model function. Thermochim Acta. 1991;188(2):333–6. doi:10.1016/0040-6031(91)87091-A.
Acknowledgements
The authors are deeply grateful to Mr. T. Yonemichi (Tokokikai Co.) for his assistance in many thermal analysis experiments and data processing. We wish to acknowledge valuable discussions and comments on the phenomenology of SCR with JAEA experts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kikuchi, S., Koga, N., Seino, H. et al. Kinetic study on liquid sodium–silica reaction for safety assessment of sodium-cooled fast reactor. J Therm Anal Calorim 121, 45–55 (2015). https://doi.org/10.1007/s10973-014-4387-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4387-x