Abstract
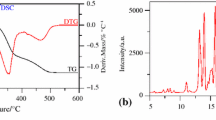
Estradiol (E2) is the main drug used in menopause therapy. This study aimed to evaluate the drug-excipient compatibility of binary mixtures (BMs) (1:1 BMs, w/w), initially by differential scanning calorimetry (DSC), and subsequently, by complementary techniques such as X-ray powder diffraction (XRPD) and high performance liquid chromatography (HPLC) if there was any evidence of interaction. The samples were stored under accelerated stability conditions (40 °C at 75 % relative humidity). The DSC curves of estradiol and the BMs with excipients (corn starch, lactose, xanthan gum, microcrystalline cellulose, magnesium stearate, dibasic calcium phosphate, and talc) were obtained. The results show that estradiol was compatible with all the selected excipients. XRPD and HPLC analysis were instrumental in interpreting the DSC results and excluding relevant pharmaceutical incompatibilities in all cases. Overall, the compatibility of the selected excipients with estradiol was successfully evaluated using a combination of thermal and spectroscopic methods, and the formulations developed using the compatible excipients were found to be stable.







Similar content being viewed by others
References
Oliveira PR, Stulzer HK, Bernardi LS, Borgmann SHM, Cardoso SG, Silva MAS. Sibutramine hydrochloride monohydrate thermal behavior, decomposition kinetics and compatibility studies. J Therm Anal Calorim. 2010;100:277–82.
Soares MFR, Soares-Sobrinho JL, Silva KER, Alves LDS, Lopes PQ, Correia LP, Souza FS, Macedo RO, Rolim-Neto PJ. Thermal characterization of antimicrobial drug ornidazole and its compatibility in a solid pharmaceutical product. J Therm Anal Calorim. 2011;104:307–13.
Soares-Sobrinho JL, Soares MFR, Lopes PQ, Correia LP, Souza FS, Macedo RO, Rolim-Neto PJA. Preformulation study of a new medicine for chagas treatment: physico-chemical characterization, thermal stability and compatibility of benznidazole. AAPS PharmSciTech. 2010;11(3):1391–6.
Tita B, Fulias A, Bandur G, Marian E, Tita D. Compatibility study between ketoprofen and pharmaceutical excipients used in solid dosage forms. J Pharm Biomed Anal. 2011;56:221–7.
Verma RK, Garg S. Selection of excipients for extended release formulations of glipizide through drug-excipient compatibility testing. J Pharm Biomed Anal. 2005;38:633–44.
Viana OS, Arauijo AAS, Simoes RA, Matos CRS, Grangeiro-Júnior S, Lima CM, Rolim-Neto PJ. Kinetic analysis of the thermal decomposition of efavirenz and compatibility studies with selected excipients. Lat Am J Pharm. 2008;27(2):211–6.
Cunha-Filho MSS, Martínez-Pacheco R, Landín M. Compatibility of the antitumoral β-lapachone with different solid dosage from excipients. J Pharm Biomed Anal. 2007;45:590–8.
Gao R, Sun BW, Lin J, Gao XL. Compatibility of medroxyprogesterone acetate and pharmaceutical excipients through thermal and spectroscopy techniques. J Therm Anal Calorim. 2014;117:731–9.
Oliveira PR, Bernardi LS, Murakami FS, Mendes C, Silva MAS. Thermal characterization and compatibility studies of norfloxacin for development of extended release tablets. J Therm Anal Calorim. 2009;97:741–5.
Lira AM, Araujo AAS, Baslio IDJ, Santos BLL, Santana DP, Macedo RO. Compatibility studies of lapachol with pharmaceutical excipients for the development of topical formulations. Thermochim Acta. 2007;457:1–6.
Tomassetti M, Catalani A, Rossi V, Vecchio S. Thermal analysis study of the interactions between acetaminophen and excipients in solid dosage forms and in some binary mixtures. J Pharm Biomed Anal. 2005;37:949–55.
Barboza F, Vecchia DD, Tagliari MP, Silva MAS, Stulzer HK. Differential scanning calorimetry as a screening technique incompatibility studies of acyclovir extended release formulations. Pharm Chem J. 2009;43:363–8.
Moyano MA, Broussalis AM, Segall AI. Thermal analysis of lipoic acid and evaluation of the compatibility with excipients. J Therm Anal Calorim. 2010;99:631–7.
Tita B, Stefanescu M, Tita D. Complex of anti-inflammatory non-steroidal drugs from carboxylic acids family. 1 Synthesis and characterization of Zn(II) complex with ibuprofen. Rev Chim (Buchar). 2011;62:1060–4.
Tita B, Stefanescu M, Tita D. Complex of anti-inflammatory non-steroidal drugs from oxicam family. 1 Synthesis and characterization of Zn(II) complex with piroxicam. Rev Chim (Buchar). 2011;62:1002–7.
Bannach G, Cervini P, Gomes Cavalheiro T, Ionashiro M. Using thermal and spectroscopic data to investigate the thermal behavior of epinephrine. Thermochim Acta. 2010;499:123–7.
El-Gamel AEN, Hawash FM, Fahmey AM. Structure characterization and spectroscopic investigation of ciprofloxacin drug. J Therm Anal Calorim. 2012;108:253–62.
Kandarapu R, Grover V, Chawla HPS. Evaluation of the compatibility of ketorolac tromethamine with selected polymers and common tablet excipients by thermal and isothermal stress testing. STP Pharm Sci. 2011;11:449–57.
Mura P, Manderioli A, Bramanti G, Furlanetto S, Pinzauti S. Utilization of differential scanning calorimetry as a screening technique of determine the compatibility of ketoprofen with excipients. Int J Pharm. 1995;11:971–9.
Luo YH, Wu GG, Sun BW. Antisolvent crystallization of Biapenem: estimation of growth and nucleation kinetics. J Chem Eng Data. 2013;58:588–97.
Araujo AAS, Storpirtis S, Mercuri LP, Carvalho FMS, Filho MS, Matos JR. Thermal analysis of the antiritroviral zidovudine (A2T) and evaluation of the compatibility with the excipients used in solid dosage forms. Int J Pharm. 2003;260:303–14.
Mura P, Manderioli A, Bramanti G, Furlanetto S, Pinzauti S. Utilization of differential scanning calorimetry as a screening technique to determine the compatibility of ketoprofen with excipients. Int J Pharm. 1995;119:71–9.
Durig T, Fassihi AR. Thermal analysis study of captopril coated tablets by thermogravimetry (TG) and differential scanning calorimetry (DSC). Int J Pharm. 1993;117:161–70.
Serajuddin AT, Thakur AB, Ghoshal RN, Fakes MG, Ranadive SA, Morris KR, Varia SA. Selection of solid dosage form composition through drug-excipient compatibility testing. J Pharm Sci. 1999;88:696–704.
Gu L, Strickley RG, Chi L, Chowhan ZT. Drug-excipient incompatibility studies of the dipeptide angiotensin-converting enzyme inhibitor, moexipril hydrochloride: dry powder vs wet granulation. Pharm Res. 1990;7:379–83.
Tita B, Fulias A, Szabadai Z, Rusu G, Bandur G, Tita D. Compatibility study between ibuprofen and excipients in their physical mixtures. J Therm Anal Calorim. 2011;105:517–27.
Gombas A, Szabo-Revesz P, Kata M, Regdon G Jr, Eros I. Quantitative determination of crystallinity of α-lactose monohydrate by DSC. J Therm Anal Calorim. 2002;68:503–10.
Desai SR, Shaikh MM, Dharwadkar SR. Preformulation compatibility studies of etamsylate and fluconazole drugs with lactoseby DSC. J Therm Anal Calorim. 2003;71:651–8.
Marini A, Berbenni V, Pegoretti M, Bruni G, Cofrancesco P, Sinistri C, Villa M. Drug-excipient compatibility studies by physico-chemical techniques. The case of atenolol. J Therm Anal Calorim. 2003;73:547–61.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91:323–8.
Acknowledgements
This work has been supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT13095), and prospective joint research project of Jiangsu province (BY2012193).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Rui Gao and Yi Jin have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Gao, R., Jin, Y., Yang, QY. et al. Study of stability and drug-excipient compatibility of estradiol and pharmaceutical excipients. J Therm Anal Calorim 120, 839–845 (2015). https://doi.org/10.1007/s10973-014-4234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4234-0