Abstract
The aim of this paper was the analytical evaluation of human milk fat substitutes (HMFS) by the calorimetric and spectroscopic methods. The HMFS were obtained by enzymatic interesterification of blend of lard or milk fat with rapeseed oil and concentrate of fish oil. The enzymatic reactions were carried out at 60, 70, and 80 °C for 2 h. A commercially immobilized 1,3-specific lipase, Lipozyme RM IM, was used as a biocatalyst. Oxidative stability of HMFS was determined using the calorimetric method. The oxidative induction time was obtained from the pressure differential scanning calorimetry curves. Peroxide value (PV) and anisidine value were determined using spectroscopic method. Interesterification caused a decrease in oxidative stability. Samples with lower induction times were characterized by higher PV. There was also a strong relation between total polar compound content and induction time. The induction times obtained for analyzed fats can be used as primary parameters for the assessment of the resistance of tested fats to their oxidative decomposition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human milk is naturally the only source of food for infants in their early life. Lipids in human milk provide a major source of energy and essential structural components for the cell membranes of the newborn. Fatty acid compositions of lipids in human milk vary with such factors as diet, lactation stage, season, and individual conditions. However, a similar general structural pattern can be seen in the corresponding triacylglycerols (TAG) of human milk [1, 2]. The fatty acid composition and structure of human milk fat (HMF) are unique. It has a high content of the saturated palmitic acid (16:0), which is primarily located at the sn-2 position of the TAG. This combination ensures a high energy content of human milk and efficient fat absorption by infants. HMF also contains long-chain polyunsaturated fatty acids (LCPUFA) at levels dependent on the dietary habits of mothers. The major LCPUFA in HMF are eicosapentaenoic acid (C20:5n-3, EPA), docosahexaenoic acid (C22:6n-3, DHA), and arachidonic acid (C20:4n-6, ARA). ARA and DHA are membrane fatty acids required for the growth and development of the central nervous system of infants. Recent studies also show the importance of ARA and DHA for bone mineralization and bone mass, probably an effect caused by the influence of the fatty acids on biosynthesis of prostaglandins. Therefore, infant formula should also contain these LCPUFA in appropriate amounts [3]. According to the EU Infant Formula and Follow-on Formulae Directive [4], LCPUFA may be added. In that case their content shall not exceed 1 % of the total fat content for n-3 LCPUFA and 2 % of the total fat content for n-6 LCPUFA (1 % of the total fat content for ARA). The European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies delivered a scientific opinion on dietary reference values for fat, including LCPUFA in 2009. The Panel proposed an adequate intake (AI) of 100 mg DHA for infants older than 6 months of age and children younger than 24 months, but it did not set any dietary reference value for ARA [5].
Developing infant milk fat similar to HMF is of great interest and a challenge to food processors [6]. According to the World Health Organization and other leading health organizations, breastfeeding is ideal, and offers specific child and maternal benefits. However, many mothers have to depend on man-made formulas to feed their babies for medical, metabolic, and economical reasons [1]. The fatty acid composition and distribution of TAG have recently gained much attention in infant formulas [7]. The current popularity on structured lipids (SL) like HMFS is well known because they are thought to improve lipids from technological or nutritional points of view. SL that contain palmitic acids predominantly at the sn-2 position of TAG and which are also enriched with DHA and EPA can be used in infant formulas to mimic the physical and chemical structure of HMF, as well as to provide the health benefits associated with the n-3 PUFA [8]. Recently, more researchers' interests have focused on resembling TAG of HMF to produce HMFS by the use of improved synthesis techniques [9]. Interesterification is one of the most important processes for modifying the physicochemical characteristics of oils and fats. During interesterification, fatty acids are exchanged within and among TAG until a thermodynamic equilibrium is reached [10, 11]. The interesterification of lipids catalyzed by lipases is an alternative to the chemical interesterification. Lipases are now widely used for the chemical redesign of fat/oil for improving physical, chemical, and/or nutritional properties [12]. In contrast to chemical methods, enzymatic synthesis as a tool for fat modification has many advantages, such as mild processing conditions, possibility of regiospecificity, and fatty acids specificity [9]. Synthesis of HMFS by enzymatic acidolysis and interesterification using specific sn-1,3 lipases are the two successful strategies that have received considerable attention in literatures [1, 9, 13].
Lard is the only fat resembling HMF in terms of fatty acid composition. Butterfat includes short-chain fatty acids and minor fatty acids whose levels are similar to those of HMF. A combination of tripalmitin or lard with vegetable oils has been chosen to obtain a product possessing similar fatty acid composition to HMF. Moreover, fish oil has been chosen to enrich with omega-3 fatty acids [14].
When developing new HMFS products, it is important not only to optimize the nutritional and physical properties, but also to ensure that the oxidative stability of the HMFS is acceptable [7]. Auto-oxidation of oils and fats is one of the most important problems in the storage and technology of food [15]. Free radicals are formed as intermediate oxidation products and then transformed into aldehydes and ketones which are responsible for undesirable flavor characteristics [16]. Many of the degradation products of edible oils and fats are harmful to human health as they destroy vitamins, inhibit enzymes, and potentially cause mutations or gastrointestinal irritations [17, 18]. Numerous methods have been developed for monitoring fat and oil auto-oxidation. Classical methods are based on “wet chemistry” [19]. Nowadays, instrumental methods are commonly used as they are faster, more precise, have wider scope of detection, and are more objective [20]. Differential scanning calorimetry (DSC) is a nonchemical method to determine fat quality parameters [17, 21–23].
The aim of this paper was the analytical evaluation of HMFS synthesized by enzymatic interesterification of blend of L and RSO with ROPUFA and blend of MF and RSO with ROPUFA by the calorimetric and spectroscopic methods.
Materials and methods
Materials
Lard, anhydrous milk fat, and rapeseed oil were provided by an industrial plant. ROPUFA 30 n-3 Food Oil (min. 30 % omega-3) was purchased in the DSM Nutritional Products company. The mixtures of L, RSO, and ROPUFA in weight proportions 7:2:1 and the mixtures of MF, RSO, and ROPUFA in weight proportions 4:5:1 were used in this investigation. Substrate proportions were determined on the basis of previous research. [24–26].
Catalyst of enzymatic interesterification
Flasks containing the initial mixtures were prepared and positioned in a thermostated mineral-oil bath shaker. After thermal equilibration of the sample at desired temperature (60, 70, or 80 °C), the enzymatic catalyst (8 % w/w) was added. The interesterifications were performed with continuous shaking and stopped by filtering off the biocatalyst after a predetermined time (2 h). As catalysts for enzymatic interesterifications, commercially prepared Lipozyme RM IM (Novozymes A/S, Bagsvaerd, Denmark) was used. Lipozyme RM IM is an immobilized lipase from Rhizomucor miehei (activity 150 IUN g−1). Reaction conditions were determined on the basis of previous research [24–26].
DSC measurements
A differential scanning calorimeter (DSC Q20 TA) coupled with a high-pressure cell (PDSC—pressure differential scanning calorimetry) was used. The instrument was calibrated using high-purity indium as a standard. Samples of fat (3–4 mg) were placed in an aluminum open pan, under oxygen atmosphere, being pressurized in an isobaric module (1,400 kPa). Experiments were performed at 120 °C under 1,400 kPa pressure of oxygen. Obtained diagrams were analyzed using TA Universal Analysis 2000 software. For each sample, measurement was triplicate, and the output was automatically recalculated and presented as amount of energy per gram. DSC conditions were determined on the basis of previous research [27].
Determination of peroxide value (PV) and anisidine value (AnV)
A modified method proposed by Hornero-Mendez et al. [28] was used for the determination of PV. Absorbance at a wavelength of 500 nm was measured. Spectrophotometer readings were set to zero using a blank sample. The concentration of lipid hydroperoxides was expressed as PV. AnV was determined spectrophotometrically in accordance with Standards EN ISO 6885:2008. The TOTOX (total oxidation) value was calculated as 2 × PV + AnV as described by Shahidi and Wanasundra [29].
Determination of free fatty acids (FFA) content
Acid values were determined by the titration of fat samples dissolved in the mixture of ethanol:diethyl ether (1:1, v/v) with 0.1 M ethanolic potassium hydroxide solution. FFA content was calculated based on acid values and the value of molar mass of oleic acid. Acid values were analyzed by the standard method ISO 660:2,000.
Determination of polar and nonpolar fractions
Fats (~2.5 g) were separated into TAG and polar fraction (PF) by column chromatography on silica gel (SG 60, 70–230 mesh, Merck, Germany). The TAG were eluted with 150 mL of mixture of petroleum ether:diethyl ether (87:13, v/v) and then the PF, that is FFA, monoacylglycerols, and diacylglycerols were eluted with 150 mL of diethyl ether. The percentages of the TAG and the PF were determined by weight after evaporation of eluting solvent. The content of polar and nonpolar compounds was analyzed by the standard method ISO 8420:2002.
GC measurements
The TAG obtained after column chromatography were analyzed for fatty acid composition. The determination of fatty acid composition was carried out by gas chromatographic (GC) analysis of fatty acid methyl esters. Methyl esters of fatty acids were prepared through saponification of TAG and esterification with methanol according to ISO 5509:2001. A YL6100 GC chromatograph equipped with a flame ionization detector and MEGA-10 capillary column of 0.25 mm i.d. × 60 m length and 0.25 μm film thickness was used. The oven temperature was programed as follows: 70 °C for 0.5 min, then it was increased by 15 °C min−1 to 160 °C; from 160 to 200 °C, it was increased by 1.5 °C min−1; and then kept at 200 °C for 15 min, from 200 to 225 °C it was increased by 30 °C, then kept at 225 °C another 1 min. The temperature of the injector was 225 °C, with a split ratio of 1:100, and the detector temperature was 250 °C. Nitrogen flowing with the rate of 1.2 mL min−1 was used as the carrier gas. The identification of fatty acids was carried out using the lipid standard purchased from Sigma Aldrich.
Statistical analysis
Microsoft Excel 2012 Software and Statgraphics Plus 5.1 were used for the statistical evaluation of the experimental data. Analysis of correlation was performed with the use of Microsoft Excel 2012 Software.
Results and discussion
Fatty acids composition
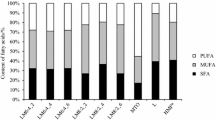
The fatty acid composition of the L, RSO, and ROPUFA and the products of interesterification as well as HMF are given in Fig. 1.
Content of fatty acids (SFA—saturated fatty acids, MUFA—monounsaturated fatty acids, PUFA—polyunsaturated fatty acids) for raw materials, for blends after interesterification and for HMF. *Values are derived from Lopez-Lopez A et al. [30]
HMF contains about 19.5 % of the polyunsaturated fatty acids (PUFA), 39.1 % of the monounsaturated fatty acids (MUFA), and 40.6 % of the saturated fatty acids (SFA) including about 14 % of the medium chain fatty acids (C8–C14) [30]. One of the objectives of this work was to obtain a specific structured HMFS product based on L or MF. Lard had a similar SFA content which is found in HMF structure, but had less PUFA content. This is confirmed by Wang et al. [9] and by Bryś et al. [31]. According to Wang et al. [9], lard has a similar palmitic and oleic acid content which is found in HMF structure, but has less medium chain fatty acids (MCFA) and essential fatty acid and has more stearic acid than those of natural HMF. Milk fat is a natural product from the dairy industry. Even though its composition and structure are significantly different from HMF, milk fat has a lot in common with mother’s milk fat in terms of content of MUFA and minor fatty acids such as conjugate linoleic acid [2, 32]. In order to enrich TAG derived from MF or L in PUFA, the enzymatic interesterification was used. As a source of PUFA, rapeseed and fish oils were used. The predominant fatty acid from RSO was oleic acid which accounted for 60.9 % of TAG. The concentrate of fish oil contained 34.5 % of the PUFA including essential DHA and EPA.
The blend of fatty acids is a key substrate for the successful production of HMFS by the interesterification method [9]. First of the blends was prepared by mixing L with RSO and ROPUFA. Second blend was prepared from MF, RSO, and ROPUFA. As shown in Fig. 1, these interesterified blends were satisfactory to produce HMFS because they have a similar ratio of PUFA to MUFA and SFA like in HMF. The unsaturated fatty acids from RSO and ROPUFA during the process interesterification were incorporated into TAG structures of L and MF. TAG of interesterified fats contained from 13.1 to 18.6 % of PUFA including important essential fatty acids from n-3 group and from n-6 group like DHA, EPA, α-linolenic acid, and linoleic acid.
FFA and PF content
The FFA and PF content of the L, MF, RSO, and ROPUFA are given in Table 1. Although RSO had the lowest FFA content, the L, MF, and ROPUFA had FFA concentrations <1 %. The FFA concentrations in the obtained HMFS were higher than those of the raw materials, indicating that the enzymatic interesterification generated the FFA. It has been found that after interesterification, the concentration of PF also increased. The main component of fat is TAG. Fats also contain certain quantities of PF namely: monoacylglycerols, diacylglycerols, and FFA. The FFA and partial acylglycerols content is associated with the activity of the lipase because the natural function of lipases is to catalyze the hydrolysis of fats. If the water level is reduced, however, some lipases will continue to catalyze reactions, and at a certain level, interesterification begins to dominate over hydrolysis [33, 34]. Blends esterified at the presence of Lipozyme RM IM contain 2.20–4.26 % of FFA and 9.23–14.24 of PF.
Oxidative stability
The successful production of SL for infant milk formulation can be impeded by their high susceptibility to oxidative deterioration [6, 35–38]. SL are TAG that have been modified to change the fatty acid composition and/or their positional distribution in the glycerol backbone by chemically and/or enzymatically catalyzed reactions and/or genetic engineering [38]. The HMFS obtained by enzymatic routes are among the most important SL for the food industry [39]. SL or infant milk produced with lipids containing unsaturated fatty acids can deteriorate during storage and produce off-flavors and odors characteristic of oxidation [6, 7, 40]. High PV value signifies a high production of primary oxidation products, which leads to faster progression of oxidation. The PV of the raw materials and SL are presented in Table 2. Interesterification causes an increase in PV. The PV of SL was about three times higher than that of the raw materials. In general, most studies have reported a decrease in oxidative stability of SL like HMFS compared to the initial fats [6, 40]. Sørensen et al. [32] have reported that the oxidative stability of the HMFS obtained from interesterified mixture of milk fat, rapeseed oil, and soybean oil was lower than that of the reference oil with the same fatty acid composition.
Therefore, the unsaturated fatty acids in HMFS can be oxidized into hydroperoxides (primary oxidation products), which can then be rapidly decomposed to secondary oxidation products such as alkanes, alkenes, aldehydes, and ketones. Oxidative deterioration can alter the nutritional quality of SL and render infant milk containing these lipids which are potentially toxic and unacceptable to consumer [6, 7, 37]. The anisidine assay is used to quantify the carbonyl compounds present in oils as a mean to determine the past history of the oil [6, 29, 37]. The raw materials had very low AnV (Table 2), in contrary to milk fat, which had an initial AnV greater than 1.0 per gram. The blends after interesterification showed an AnV lower than 2 per gram. The SL obtained from blend of MF, RSO, and ROPUFA were characterized by higher values of AnV than the SL obtained from blend of L, RSO, and ROPUFA. The TOTOX value is a combination of the PV and AnV normally used to determine the total oxidative stability of the oil [6]. The SL had higher TOTOX values than the raw materials (Table 2) that make them less stable to oxidation.
DSC measurements
DSC is one of the fastest and the most convenient methods for the assessment of the oxidative stability of oils and fats. There are reports saying that the DSC data for vegetable oil oxidation correlate well with Rancimat and electron spin resonance (ESR) spectroscopy results [27, 41–44]. The results of PDSC measurements expressed as the oxidation induction times are shown in Fig. 2. The PDSC tests for interesterified fats performed in isothermal temperature 120 °C showed that their induction times were reduced compared with the raw materials. The induction times obtained for analyzed fats can be used as primary parameters for the assessment of the resistance of tested fats to their oxidative decomposition. Generally, samples with longer induction times are more stable than those for which the induction time obtained at the same temperature is shorter [34]. Most studies have reported a decrease in oxidative stability of interesterified fats compared to the initial mixture [6, 31, 40].
Most studies have suggested the loss of antioxidants during the production of SL as the main reason for explaining a worse oxidative stability found for SL, even regardless of the production method used or of the polyunsaturated nature of fatty acids. This loss of endogenous antioxidants is especially important when vegetable oils are used as a source of fatty acids for the production of SL, due to their natural content in antioxidants, such as tocols, both tocopherols and tocotrienols, phytosterols, or phenolic compounds [40]. Ledochowska and Wilczynska [45] found that enzymatic interesterification reduced the level of tocopherols from 28.8 mg per 100 g to 16.2 mg per 100 g.
Analyzing of correlation for induction time, PF, and PV
The results obtained for the oxidative stability and for the DSC measurements indicate an inverse relation between PV and induction time. An inverse relation between PF and induction time was also observed. Increased content of PF in the interesterified fatty product can reduce its resistance to oxidation [34].
One component of the PF is the FFA. Hamam and Shahidi [46] suggested that the presence of FFAs in the reaction mixture may induce oxidation due to a catalytic effect of the carboxylic groups of the FFA on the formation of free radicals. In general, the higher the level of FFAs, monoacylglycerols and diacylglycerols in the obtained product after interesterification with respect to the level of TAG, the higher the reduced oxidative stability [40]. The analysis of correlation was conducted for PV and the content of total polar compounds. Data set consisted of 10 samples; one sample was identified as an outlier. Statistically significant (p value <0.05) correlations were found for PV as well as for total polar compound content (Figs. 3 and 4). The values of R 2 (coefficient of determination) above 0.9 indicated that there is a strong relation between those parameters and induction time. Obtained relations will be further described in the future studies including higher amount of samples.
Conclusions
The interesterified blend of L and RSO with ROPUFA and blend of MF and RSO with ROPUFA were satisfactory to produce HMFS because they have a similar ratio of the PUFA to the MUFA and to the SFA like in HMF. Both the calorimetric methods and spectroscopic methods are fast and reliable methods, which can be used to assess the oxidation parameters of SL. There is a strong relation between total polar compound content and induction time as well as between PV and induction time. Obtained correlations can be used for the evaluation of oxidative stability of unknown oil sample.
Abbreviations
- L:
-
Lard
- MF:
-
Milk fat
- RSO:
-
Rapeseed oil
- ROPUFA:
-
Concentrate of fish oil
- L60_2:
-
Mixture of L, RSO and ROPUFA in proportions 72:1, interesterified for 2 h at 60 °C
- L70_2:
-
Mixture of L, RSO and ROPUFA in proportions 72:1, interesterified for 2 h at 70 °C
- L80_2:
-
Mixture of L, RSO and ROPUFA in proportions 72:1, interesterified for 2 h at 80 °C
- M60_2:
-
Mixture of MF, RSO and ROPUFA in proportions 45:1, interesterified for 2 h at 60 °C
- M70_2:
-
Mixture of MF, RSO and ROPUFA in proportions 45:1, interesterified for 2 h at 70 °C
- M80_2:
-
Mixture of MF, RSO and ROPUFA in proportions 45:1, interesterified for 2 h at 80 °C
- HMF:
-
Human milk fat
References
Yang T, Xu X, He C, Li L. Lipase-catalyzed modification of lard to produce human milk fat substitutes. Food Chem. 2003;80:473–81.
Jensen RG. The lipids in human milk. Prog Lipid Res. 1996;35:53–92.
Huiling M. Production and nutritional aspects of human milk fat substitutes. Lipid Technol. 2010;22:126–9.
EU Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae.
EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010;8:1461.
Maduko C, Park Y, Akoh CC. Characterization and oxidative stability of structured lipids: infant milk fat analog. JAOCS. 2008;85:197–204.
Nielsen NS, Yang T, Xu X, Jacobsen Ch. Production and oxidative stability of a human milk fat substitute produced from lard by enzyme technology in a pilot packed-bed reactor. Food Chem. 2006;94:53–60.
Sahin N, Akoh CC, Karaali A. Human milk fat substitutes containing omega-3 fatty acids. J Agric Food Chem. 2006;54:3717–22.
Wang YH, Qin XL, Zhu QS, Zhou R, Yang B, Li L. Lipase-catalyzed acidolysis of lard for the production of human milk fat substitute. Eur Food Res Technol. 2010;230:769–77.
Karabulut I, Turan S, Ergin G. Effects of chemical interesterification on solid fat content and slip melting point of fat/oil blends. Eur Food Res Technol. 2004;218:224–9.
Habi Mat Dian NL, Sundram K, Idris NA. DSC study on the melting properties of palm oil, sunflower oil, and palm kernel olein blends before and after chemical interesterification. JAOCS. 2006;83:739–45.
Karabulut I, Turan S, Vural H, Kayahan M. Production of human milk fat substitute. Food Technol Biotechnol. 2007;45:434–8.
Maduko CO, Akoh CC, Park YW. Enzymatic interesterification of tripalmitin with vegetable oil blend for formulation of caprine milk infant formula analogs. J Dairy Sci. 2007;90:594–601.
Ilyasoglu H. Production of human fat milk analogue containing α-linolenic acid by solvent-free enzymatic interesterification. LWT—Food Sci Technol. 2013;54:179–85.
Kasprzycka-Guttman T, Jarosz-Jarszewska M, Litwinienko G. Specific heat and kinetic parameters of thermo-oxidative decomposition of peanut oil. Thermochim Acta. 1995;250:197–205.
Thurgood J, Ward R, Martini S. Oxidation kinetics of soybean oil/anhydrous milk fat blends: a differential scanning calorimetry study. Food Res Int. 2007;40:1030–7.
Tan CP, Che Man YB. Differential scanning calorimetric analysis for monitoring the oxidation of heated oils. Food Chem. 1999;67:177–84.
Clark WL, Serbia, GW. Safety aspects of frying fats and oils. Food Tech. 1991;45:84–86, 88, 89, 94.
Gray JI. Measurement of lipid oxidation: a review. JAOCS. 1978;55:539–46.
Ostrowska-Ligęza E, Bekas W, Kowalska D, Lobacz M, Wroniak M, Kowalski B. Kinetics of commercial olive oil oxidation: dynamic differential scanning calorimetry and Rancimat studies. Eur J Lipid Sci Technol. 2010;112:268–74.
Ostrowska-Ligęza E, Górska A, Wirkowska M, Koczoń P. An assessment of various powdered baby formulas by conventional methods (DSC) or FT-IR spectroscopy. J Therm Anal Calorim. 2012;110:465–71.
Górska A, Ostrowska-Ligęza E, Szulc K, Wirkowska M. A differential scanning calorimetric study of β-lactoglobulin and vitamin D3 complexes. J Therm Anal Calorim. 2012;110:473–7.
Górska A, Szulc K, Ostrowska-Ligęza E, Wirkowska M, Bryś J. The influence of trehalose–maltodextrin and lactose–maltodextrin matrices on thermal and sorption properties of spray-dried β-lactoglobulin–vitamin D3 complexes. J Therm Anal Calorim. 2013;112:429–36.
Tarnowska K, Bryś J, Wirkowska M, Gruczyńska E. Lipidy strukturyzowane bogate w kwasy omega-3 otrzymane na drodze enzymatycznego przeestryfikowania smalcu. Postępy Techniki Przetwórstwa Spożywczego. 2011;21:45–51.
Wirkowska M, Bryś J, Górska A, Ostrowska-Ligęza E, Tarnowska K. Próba wzbogacania tłuszczu mlecznego kwasami EPA i DHA. Żywność. Nauka. Technologia. Jakość. 2012;82:46–55.
Wirkowska M, Bryś J, Górska A, Ostrowska-Ligęza E, Żubrżycka K. Wzbogacanie tłuszczu mlecznego w kwasy n-3 na drodze przeestryfikowania enzymatycznego. Bromatologia i Chemia Toksykologiczna. 2012;45:699–704.
Kowalski B, Ratusz K, Kowalska D, Bekas W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. Eur J Lipid Sci Technol. 2004;106:16–165.
Hornero-Mendez D, Perez-Galvez A, Minguez-Mosquera MIA. A rapid spectrophotometric method for the determination of peroxide value in food lipids with high carotenoid content. JAOCS. 2001;78:1151–5.
Shahidi F, Wanasundra UN. Methods for measuring oxidative rancidity in fats and oils. In: Akoh CC, Min DB, editors. Food lipids, chemistry, nutrition and biotechnology. 2nd ed. New York: Marcel Dekker; 2002. p. 465–87.
Lopez-Lopez A, Castellote-Bargalló AI, Campoy-Folgoso C, Rivero-Urgel M, Lopez-Sabater MC. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and infant formulas. European J Clin Nutr. 2002;56:1242–54.
Bryś J, Wirkowska M, Górska A, Ostrowska-Ligęza E, Bryś A, Koczoń P. The use of DSC and FT-IR spectroscopy for evaluation of oxidative stability of interesterified fats. J Therm Anal Calorim. 2013;113:481–7.
Sørensen A-DM, Xu X, Zhang L, Kristensen JB, Jacobsen C. Human milk fat substitute from butterfat: production by enzymatic interesterification and evaluation of oxidative stability. JAOCS. 2010;87:185–94.
Liu L, Lampert D. Monitoring chemical interesterification. JAOCS. 1999;76:783–7.
Kowalski B, Tarnowska K, Gruczynska E, Bekas W. Chemical and enzymatic interesterification of beef tallow and rapeseed oil blend with low content of tallow. J Oleo Sci. 2004;53(10):479–88.
Jennings BH, Akoh CC. Enzymatic modification of triacylglycerols of high eicosapentaenoic and docosahexaenoic acids content to produce structured lipids. JAOCS. 1999;76:1133–7.
Akoh CC, Moussata CO. Characterization and oxidative stability of enzymatically produced fish and canola oil-based structured lipids. JAOCS. 2001;78:25–30.
Osborn HT, Akoh CC. Effect of emulsifier type, droplet size, and oil concentration on lipid oxidation in structured lipid- based oil-in-water emulsions. Food Chem. 2003;84:451–6.
Claro da Silva R, Schaffer De Martini Soares FA, Hazzan M, Capacla IR, Almeida Gonçalves MI, Gioielli LA. Continuous enzymatic interesterification of lard and soybean oil blend: effects of different flow rates on physical properties and acyl migration. J Mol Cat B. 2012;76:23–8.
Tecelao C, Silva J, Dubreucq E, Ribeiro M, Ferreira-Dias S. Production of human milk fat substitutes enriched in omeg-3 polyunsaturated fatty acids using immobilized commercial lipases and Candida parapsilosis lipase/acyltransferase. J Mol Cat B. 2010;65:122–7.
Martin D, Reglero G, Senorans FJ. Oxidative stability of structured lipids. Eur Food Res Technol. 2010;231:635–53.
Kowalski B, Gruczynska E, Maciaszek K. Kinetics of rapeseed oil oxidation by pressure differential scanning calorimetry measurements. Eur J Lipid Sci Technol. 2000;102:337–41.
Tan CP, Che Man YB. Recent developments in differential scanning calorimetry for assessing oxidative deterioration of vegetable oils. Trends Food Sci Technol. 2002;13:312–8.
Wirkowska M, Ostrowska-Ligęza E, Górska A, Koczoń P. Thermal properties of fats extracted from powdered baby formulas. J Therm Anal Calorim. 2012;110:137–43.
Velasco J, Andersen ML, Skibsted LH. Evaluation of oxidative stability of vegetable oils by monitoring the tendency to radical formation. A comparison of electron spin resonance spectroscopy with the Rancimat method and differential scanning calorimetry. Food Chem. 2004;85:624–32.
Ledochowska E, Wilczynska E. Comparison of the oxidative stability of chemically and enzymatically interesterified fats. Fett/Lipid. 1998;100:343–8.
Hamam F, Shahidi F. Enzymatic acidolysis of an arachidonic acid single-cell oil with capric acid. JAOCS. 2004;81:887–92.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education Grant No. N N312 068439.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bryś, J., Wirkowska, M., Górska, A. et al. Application of the calorimetric and spectroscopic methods in analytical evaluation of the human milk fat substitutes. J Therm Anal Calorim 118, 841–848 (2014). https://doi.org/10.1007/s10973-014-3893-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3893-1