Abstract
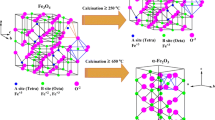
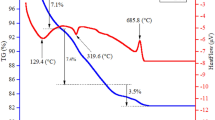
Thermal behavior of highly crystalline ε-Fe2O3 nanoparticles of different apparent crystallite sizes was characterized using thermogravimetry, differential thermal analysis, and analysis of evolved gas by mass spectrometry. Phase composition of the samples was monitored ex situ by X-ray powder diffraction. The results show that the thermal stability of this metastable iron oxide polymorph decreases with increasing particle size. For the particle diameter of 19(2) nm, the transformation temperature was equal to 794(5) °C, while for 28(2) nm only 755(10) °C. Surface of the nanoparticles contained adsorbed water and carbon dioxide. Elimination of these species proceeds in two steps. Water is removed at temperatures below 200 °C and CO2 in the temperature range between 200 and 450 °C.




Similar content being viewed by others
References
Jin J, Ohkoshi SI, Hashimoto K. Giant coercive field of nanometer-sized iron oxide. Adv Mater. 2004;16:48–51.
Sakurai S, Shimoyama JI, Hashimoto K, Ohkoshi SI. Large coercive field in magnetic-field oriented ε-Fe2O3 nanorods. Chem Phys Lett. 2008;458:333–6.
Gich M, Gazquez J, Roig A, Crespi A, Fontcuberta J, Idrobo JC, Pennycook SJ, Varela M, Skumryev V, Varela M. Epitaxial stabilization of ε-Fe2O3 (00l) thin films on SrTiO3 (111). Appl Phys Lett. 2010;96:112508.
Kryder MH, Gage EC, McDaniel TW, Challenger WA, Rottmayer RE, Ju G, Hsia YT, Erden MF. Heat assisted magnetic recording. Proc IEEE. 2008;96:1810–34.
Namai A, Sakurai S, Nakajima M, Suemoto T, Matsumoto K, Goto M, Sasaki S, Ohkoshi SI. Synthesis of an electromagnetic wave absorber for high-speed wireless communication. J Am Chem Soc. 2009;131:1170–3.
Ohkoshi SI, Kuroki S, Sakurai S, Matsumoto K, Sato K, Sasaki S. A millimeter-wave absorber based on gallium-substituted ε-iron oxide nanomagnets. Angew Chem Int Ed. 2007;46:8392–5.
Namai A, Yoshikiyo M, Yamada K, Sakurai S, Goto T, Yoshida T, Miyazaki T, Nakajima M, Suemoto T, Tokoro H, Ohkoshi SI. Hard magnetic ferrite with a gigantic coercivity and high frequency millimetre wave rotation. Nature Commun. 2012;3:1035.
Tronc E, Chanéac C, Jolivet JP. Structural and magnetic characterization of ε-Fe2O3. J Solid State Chem. 1998;139:93–104.
Popovici M, Gich M, Nižňanský D, Roig A, Savii C, Casas L, Molins E, Zaveta K, Enache C, Sort J, de Brion S, Chouteau G, Nogués J. Optimized synthesis of the elusive ε-Fe2O3 phase via sol–gel chemistry. Chem Mater. 2004;16:5542–8.
Barick KC, Varaprasad BSDChS, Bahadur D. Structural and magnetic properties of γ- and ε-Fe2O3 nanoparticles dispersed in silica matrix. J Non-Cryst Solids. 2010;356:153–9.
Jin J, Hashimoto K, Ohkoshi SI. Formation of spherical and rod-shaped ε-Fe2O3 nanocrystals with a large coercive field. J Mater Chem. 2005;15:1067–71.
Brázda P, Nižňanský D, Rehspringer JL, Poltierová Vejpravová J. Novel sol–gel method for preparation of high concentration ε-Fe2O3/SiO2 nanocomposite. J Sol–Gel Sci Technol. 2009;51:78–83.
Nakamura T, Yamada Y, Yano K. Novel synthesis of highly monodispersed γ-Fe2O3/SiO2 and ε-Fe2O3/SiO2 nanocomposite spheres. J Mater Chem. 2006;16:2417–9.
Sakurai S, Namai A, Hashimoto K, Ohkoshi SI. First observation of phase transformation of all four Fe2O3 phases (γ - > ε- > β- > α-phase). J Am Chem Soc. 2009;131:18299–303.
Delahaye E, Escax V, El Hassan N, Davidson A, Aquino R, Dupuis V, Perzynski R, Raikher YL. “Nanocasting”: using SBA-15 silicas as hard templates to obtain ultrasmall monodispersed γ-Fe2O3 nanoparticles. J Phys Chem B. 2006;110:26001–11.
Ohkoshi SI, Sakurai S, Jin J, Hashimoto K. The addition effects of alkaline earth ions in the chemical synthesis of ε-Fe2O3 nanocrystals that exhibit a huge coercive field. J Appl Phys. 2005;97:10K312.
Gich M, Roig A, Taboada E, Molins E, Bonafos C, Snoeck E. Stabilization of metastable phases in spatially restricted fields: the case of the Fe2O3 polymorphs. Faraday Discuss. 2007;136:345–54.
Taboada E, Gich M, Roig A. Nanospheres of silica with an ε-Fe2O3 single crystal nucleus. ACS Nano. 2009;3:3377–82.
Trautmann JM, Forestier H. Nouvelle préparation et étude de l’oxyde ε-Fe2O3. C R Acad Sci (Paris). 1965;261:4423–5.
Dézsi I, Coey JMD. Magnetic and thermal properties of ε-Fe2O3. Phys Stat Sol A. 1973;15:681–5.
Zboril R, Mashlan M, Barcova K, Vujtek M. Thermally induced solid-state syntheses of γ-Fe2O3 nanoparticles and their transformation to α-Fe2O3 via ε-Fe2O3. Hyperfine Interact. 2002;139(140):597–606.
Rodriguez-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys B. 1993;192:55–69.
Gich M, Frontera C, Roig A, Taboada E, Molins E, Rechenberg HR, Ardisson JD, Macedo WAA, Ritter C, Hardy V, Sort J, Skumryev V, Nogués J. High- and low-temperature crystal and magnetic structures of ε-Fe2O3 and their correlation to its magnetic properties. Chem Mater. 2006;18:3889–97.
Maslen EN, Streltsov VA, Streltsova NR, Ishizawa N. Synchrotron Xray study of the electron density in α-Fe2O3. Acta Cryst. 1994;B50:435–41.
Ben-Dor L, Fischbein E, Kalman ZH. Concerning the β phase of iron(III) oxide. Acta Cryst. 1976;B32:667.
Jørgensen JE, Mosegaard L, Thomsen LE, Jensen TR, Hanson JC. Formation of γ-Fe2O3 nanoparticles and vacancy ordering: an in situ X-ray powder diffraction study. J Solid State Chem. 2007;180:180–5.
Šubrt J, Balek V, Criado JM, Pérez-Maqueda LA, Večerníková E. Characterisation of α-FeOOH grinding products using simultaneous DTA and TG/DTG coupled with MS. J Therm Anal Calorim. 1998;53:509–17.
Maciejewski M, Baiker A. Quantitative calibration of mass spectrometric signals measured in coupled TA-MS. Thermochim Acta. 1997;295:95–105.
Baltrusaitis J, Schuttlefield J, Zeitler E, Grassian VH. Carbon dioxide adsorption on oxide nanoparticles. Chem Eng J. 2011;170:471–81.
Machala L, Tuček J, Zbořil R. Polymorphous transformations of nanometric iron(III) oxide: a review. Chem Mater. 2011;23:3255–72.
Belonoshko AB, Ahuje R, Johansson B. Mechanism for κ-Al2O3 to the α-Al2O3 transition and the stability of κ-Al2O3 under volume expansion. Phys Rev B. 2000;61:3131–4.
Acknowledgements
Dr. Mariana Klementová is acknowledged for careful reading the manuscript. This work was supported by the Czech Science Foundation, project no. P204/10/0035 and the Long-Term Research Plan of the Ministry of Education of the Czech Republic (MSM0021620857).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brázda, P., Večerníková, E., Pližingrová, E. et al. Thermal stability of nanocrystalline ε-Fe2O3 . J Therm Anal Calorim 117, 85–91 (2014). https://doi.org/10.1007/s10973-014-3711-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3711-9