Abstract
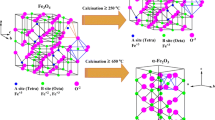
Evolution of the local environment of Fe3+ ions in deposited Fe2O3/SiO2 nanoparticles formed in samples with different iron contents was investigated in order to establish the conditions for obtaining the stable ε-Fe2O3/SiO2 samples without impurities of other iron oxide polymorphs. Microstructure of the samples with an iron content of up to 16% is studied by high-resolution transmission electron microscopy, X-ray diffraction analysis, and Mössbauer spectroscopy, and their magnetic properties are examined. At iron concentrations below 6%, calcinations of iron-containing precursor nanoparticles in a silica gel matrix lead to the formation of the ε-Fe2O3 iron oxide polymorphic modification without foreign phase impurities, while at the iron concentration in the range of 6–12%, the hematite phase forms in the sample in the fraction of no more than 5%. It is concluded on the basis of the data obtained that the spatial stabilization of iron-containing particles is one of the main factors facilitating the formation of the ε-Fe2O3 phase in a silica gel matrix without other iron oxide polymorphs. It is demonstrated that the increase in the iron content leads to the formation of larger particles in the sample and gradual changes of the Fe3+ ion local environment during the phase transition ε-Fe2O3 → α-Fe2O3.







Similar content being viewed by others
References
Chu, Y., Pan, Q.: Three-dimensionally macroporous Fe/C nanocomposites as highly selective oil-absorption materials. ACS Appl. Mater. Interfaces. 4, 2420–2425 (2012). https://doi.org/10.1021/am3000825
Somorjai, G.A., McCrea, K.: Roadmap for catalysis science in the 21st century: a personal view of building the future on past and present accomplishments. Appl. Catal. A Gen. 222, 3–18 (2001). https://doi.org/10.1016/S0926-860X(01)00825-0
Shuvaeva, M.A., Nuzhdin, A.L., Martyanov, O.N., Bukhtiyarova, G.A.: Benzylation of benzene by benzyl chloride over silica-supported iron sulfate catalysts. Mendeleev Commun. 24, 231–232 (2014). https://doi.org/10.1016/j.mencom.2014.06.015
Booker, N.A., Keir, D., Priestley, A.J., Ritchie, C.B., Sudarmana, D.L., Woods, M.A.: Sewage clarification with magnetite particles. Water Sci. Technol. 23, 1703–1712 (1991)
Seil, J.T., Webster, T.J.: Antimicrobial applications of nanotechnology: methods and literature. Int. J. Nanomedicine. 7, 2767–2781 (2012). https://doi.org/10.2147/IJN.S24805
Gupta, A.K., Gupta, M.: Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26, 3995–4021 (2005). https://doi.org/10.1016/j.biomaterials.2004.10.012
Lu, A.-H., Salabas, E.L., Schüth, F.: Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew. Chemie Int. Ed. 46, 1222–1244 (2007). https://doi.org/10.1002/anie.200602866
Sakurai, S., Kuroki, S., Tokoro, H., Hashimoto, K., Ohkoshi, S.: Synthesis, crystal structure, and magnetic properties of ε-InxFe2–xO3 nanorod-shaped magnets. Adv. Funct. Mater. 17, 2278–2282 (2007). https://doi.org/10.1002/adfm.200600581
Tronc, E., Chanéac, C., Jolivet, J.P.: Structural and magnetic characterization of ε-Fe2O3. J. Solid State Chem. 139, 93–104 (1998). https://doi.org/10.1088/0953-8984/23/12/126003
Gich, M., Roig, A., Taboada, E., Molins, E., Bonafos, C., Snoeck, E.: Stabilization of metastable phases in spatially restricted fields: the case of the Fe2O3 polymorphs. Faraday Disc. 136, 345 (2007). https://doi.org/10.1039/b616097b
Sakurai, S., Namai, A., Hashimoto, K., Ohkoshi, S.-I.: First observation of phase transformation of all four Fe2O3 phases (γ →ε →β →α-phase). J. Am. Chem. Soc. 131, 18299–18303 (2009). https://doi.org/10.1021/ja9046069
Jin, J., Ohkoshi, S., Hashimoto, K.: Giant coercive field of nanometer-sized iron oxide. Adv. Mater. 16, 48–51 (2004). https://doi.org/10.1002/adma.200305297
Sakurai, S., Jin, J., Hashimoto, K., Ohkoshi, S.: Reorientation phenomenon in a magnetic phase of ε-Fe2O3 nanocrystal. J. Phys. Soc. Japan 74, 1946–1949 (2005). https://doi.org/10.1143/JPSJ.74.1946
Gich, M., Roig, A., Frontera, C., Molins, E., Sort, J., Popovici, M., Chouteau, G., Martín y Marero, D., Nogués, J.: Large coercivity and low-temperature magnetic reorientation in ε-Fe2O3 nanoparticles. J. Appl. Phys. 98, 44307 (2005). https://doi.org/10.1063/1.1997297
Tseng, Y.C., Souza-Neto, N.M., Haskel, D., Gich, M., Frontera, C., Roig, A., Van Veenendaal, M., Nogués, J.: Nonzero orbital moment in high coercivity ε-Fe2O3 and low-temperature collapse of the magnetocrystalline anisotropy. Phys. Rev. B - Condens. Matter Mater. Phys. 79, 1–6 (2009). https://doi.org/10.1103/PhysRevB.79.094404
Yakushkin, S.S., Bukhtiyarova, G.A., Martyanov, O.N.: Formation conditions of a magnetically ordered phase ε-Fe2O3. A FMR in situ study. J. Struct. Chem. 54, 876–882 (2013). https://doi.org/10.1134/S0022476613050065
Zboril, R., Mashlan, M., Barcova, K., Vujtek, M.: Thermally induced solid-state syntheses of γ-Fe2O3 nanoparticles and their transformation to α-Fe2O3 via ε-Fe2O3. Hyperfine Interact. 139(140), 597–606 (2002). https://doi.org/10.1023/A:1021226929237
Ding, Y., Morber, J.R., Snyder, R.L., Wang, Z.L.: Nanowire structural evolution from Fe3O4 to ε-Fe2O3. Adv. Funct. Mater. 17, 1172–1178 (2007). https://doi.org/10.1002/adfm.200601024
Tadić, M., Spasojević, V., Kusigerski, V., Marković, D., Remškar, M.: Formation of ε-Fe2O3 phase by the heat treatment of α-Fe2O3/SiO2 nanocomposite. Scr. Mater. 58, 703–706 (2008). https://doi.org/10.1016/j.scriptamat.2007.12.009
Popovici, M., Gich, M., Nižňanský, D., Roig, A., Savii, C., Casas, L., Molins, E., Zaveta, K., Enache, C., Sort, J., de Brion, S., Chouteau, G., Nogués, J.: Optimized synthesis of the elusive ε-Fe2O3 phase via sol −gel chemistry. Chem. Mater. 16, 5542–5548 (2004). https://doi.org/10.1021/cm048628m
Sakurai, S., Shimoyama, J.I., Hashimoto, K., Ohkoshi, S.I.: Large coercive field in magnetic-field oriented -Fe2O3 nanorods. Chem. Phys. Lett. 458, 333–336 (2008). https://doi.org/10.1016/j.cplett.2008.04.121
Tuček, J., Zbořil, R., Namai, A., Ohkoshi, S.: ε-Fe2O3: an advanced nanomaterial exhibiting giant coercive field, millimeter-wave ferromagnetic resonance, and magnetoelectric coupling. Chem. Mater. 22, 6483–6505 (2010). https://doi.org/10.1021/cm101967h
Ohkoshi, S., Sakurai, S., Jin, J., Hashimoto, K.: The addition effects of alkaline earth ions in the chemical synthesis of ε-Fe2O3 nanocrystals that exhibit a huge coercive field. J. Appl. Phys. 97, 10K312 (2005). https://doi.org/10.1063/1.1855615
Sakurai, S., Tomita, K., Hashimoto, K., Yashiro, H., Ohkoshi, S.: Preparation of the nanowire form of ε-Fe2O3 single crystal and a study of the formation process. J. Phys. Chem. C. 112, 20212–20216 (2008). https://doi.org/10.1021/jp806336f
Lee, S., Xu, H.: Size-dependent phase map and phase transformation kinetics for nanometric iron(III) oxides (γ →ε →α pathway). J. Phys. Chem. C. 120, 13316–13322 (2016). https://doi.org/10.1021/acs.jpcc.6b05287
El Mendili, Y., Bardeau, J.-F., Randrianantoandro, N., Greneche, J.-M., Grasset, F.: Structural behavior of laser-irradiated γ-Fe2O3 nanocrystals dispersed in porous silica matrix: γ-Fe2O3 to α-Fe2O3 phase transition and formation of ε-Fe2O3. Sci. Technol. Adv. Mater. 17, 597–609 (2016). https://doi.org/10.1080/14686996.2016.1222494
Nikolic, V.N., Spasojevic, V., Panjan, M., Kopanja, L., Mrakovic, A., Tadic, M.: Re-formation of metastable ε-Fe2O3 in post-annealing of Fe2O3/SiO2 nanostructure: synthesis, computational particle shape analysis in micrographs and magnetic properties. Ceram. Int. 43, 7497–7507 (2017). https://doi.org/10.1016/j.ceramint.2017.03.030
Nikolić, V.N., Tadić, M., Panjan, M., Kopanja, L., Cvjetićanin, N., Spasojević, V.: Influence of annealing treatment on magnetic properties of Fe2O3/SiO2 and formation of ε-Fe2O3 phase. Ceram. Int. 43, 3147–3155 (2017). https://doi.org/10.1016/j.ceramint.2016.11.132
Bukhtiyarova, G. A., Mart’yanov, O.N., Yakushkin, S.S., Shuvaeva, M. A., Bayukov, O. A.: State of iron in nanoparticles prepared by impregnation of silica gel and aluminum oxide with FeSO4 solutions. Phys. Solid State 52, 826–837 (2010). https://doi.org/10.1134/S1063783410040268
Bukhtiyarova, G.A., Shuvaeva, M.A., Bayukov, O.A., Yakushkin, S.S., Martyanov, O.N.: Facile synthesis of nanosized ε-Fe2O3 particles on the silica support. J. Nanoparticle Res. 13, 5527–5534 (2011). https://doi.org/10.1007/s11051-011-0542-5
Yakushkin, S.S., Dubrovskiy, A.A., Balaev, D.A., Shaykhutdinov, K.A., Bukhtiyarova, G.A., Martyanov, O.N.: Magnetic properties of few nanometers ε-Fe2O3 nanoparticles supported on the silica. J. Appl. Phys. 111, 44312 (2012). https://doi.org/10.1063/1.3686647
Balaev, D.A., Dubrovskiy, A.A., Shaykhutdinov, K.A., Bayukov, O.A., Yakushkin, S.S., Bukhtiyarova, G.A., Martyanov, O.N.: Surface effects and magnetic ordering in few-nanometer-sized ε-Fe2O3 particles. J. Appl. Phys. 114, 163911 (2013). https://doi.org/10.1063/1.4827839
Balaev, D.A., Poperechny, I.S., Krasikov, A.A., Shaikhutdinov, K.A., Dubrovskiy, A.A., Popkov, S.I., Balaev, A.D., Yakushkin, S.S., Bukhtiyarova, G.A., Martyanov, O.N., Raikher, Y.L.: Dynamic magnetization of ε-Fe2O3 in pulse field: evidence of surface effect. J. Appl. Phys. 117, 63908 (2015). https://doi.org/10.1063/1.4907586
Balaev, D.A., Yakushkin, S.S., Dubrovskii, A.A., Bukhtiyarova, G.A., Shaikhutdinov, K.A., Martyanov, O.N.: Study of the high-coercivity material based on ε-Fe2O3 nanoparticles in the silica gel matrix. Tech. Phys. Lett. 42, 347–350 (2016). https://doi.org/10.1134/S1063785016040039
Dubrovskiy, A.A., Balaev, D.A., Shaykhutdinov, K.A., Bayukov, O.A., Pletnev, O.N., Yakushkin, S.S., Bukhtiyarova, G.A., Martyanov, O.N.: Size effects in the magnetic properties of ε-Fe2O3 nanoparticles. J. Appl. Phys. 118, 213901 (2015). https://doi.org/10.1063/1.4936838
Amin, N., Arajs, S.: Morin temperature of annealed submicronic α-F2O3 particles. Phys. Rev. B. 35, 4810–4811 (1987). https://doi.org/10.1103/PhysRevB.35.4810
Mørup, S., Madsen, D.E., Frandsen, C., Bahl, C.R.H., Hansen, M.F.: Experimental and theoretical studies of nanoparticles of antiferromagnetic materials. J. Phys. Condens. Matter. 19, 213202 (2007). https://doi.org/10.1088/0953-8984/19/21/213202
Acknowledgments
The work was supported by the Russian Science Foundation (Grant No. 17-12-01111).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yakushkin, S.S., Balaev, D.A., Dubrovskiy, A.A. et al. Evolution of the Fe3+ Ion Local Environment During the Phase Transition ε-Fe2O3 → α-Fe2O3 . J Supercond Nov Magn 31, 1209–1217 (2018). https://doi.org/10.1007/s10948-017-4307-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-017-4307-y