Abstract
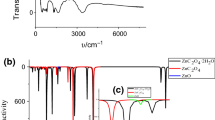
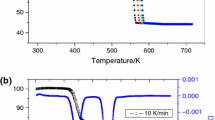
This study is devoted to the thermal decomposition of ZnC2O4·2H2O, which was synthesized by solid-state reaction using C2H2O4·2H2O and Zn(CH3COO)2·2H2O as raw materials. The initial samples and the final solid thermal decomposition products were characterized by Fourier transform infrared and X-ray diffraction. The particle size of the products was observed by transmission electron microscopy. The thermal decomposition behavior was investigated by thermogravimetry, derivative thermogravimetric and differential thermal analysis. Experimental results show that the thermal decomposition reaction includes two stages: dehydration and decomposition, with nanostructured ZnO as the final solid product. The Ozawa integral method along with Coats–Redfern integral method was used to determine the kinetic model and kinetic parameters of the second thermal decomposition stage of ZnC2O4·2H2O. After calculation and comparison, the decomposition conforms to the nucleation and growth model and the physical interpretation is summarized. The activation energy and the kinetic mechanism function are determined to be 119.7 kJ mol−1 and G(α) = −ln(1 – α)1/2, respectively.








Similar content being viewed by others
References
Dolan MD, Ilyushechkin AY, McLennan KG, Nguyen T, Sharma SD. Glass-based processing of mixed-oxide desulfurization sorbents. Ind Eng Chem Res. 2009;48:10498–503.
Fan HL, Li YX, Li CH, Guo HX, Xie KC. The apparent kinetics of H2S removal by zinc oxide in the presence of hydrogen. Fuel. 2002;81:91–6.
Novochinskii II, Song CS, Ma XL, Liu XS, Shore L, Lampert J, Farrauto RJ. Low-temperature H2S removal from steam-containing gas mixtures with ZnO for fuel cell application. 1. ZnO particles and extrudates. Energy Fuels. 2004;18:576–83.
Yang HY, Sothen R, Cahela DR, Tatarchuk BJ. Breakthrough characteristics of reformate desulfurization using ZnO sorbents for logistic fuel cell power systems. Ind Eng Chem Res. 2008;47:10064–70.
Ling LX, Zhang RG, Han PD, Wang BJ. DFT study on the sulfurization mechanism during the desulfurization of H2S on the ZnO desulfurizer. Fuel Process Technol. 2013;106:222–30.
Rodriguez JA, Maiti A. Adsorption and decomposition of H2S on MgO(100), NiMgO(100), and ZnO(0001) surfaces: a first-principles density functional study. J Phys Chem B. 2000;104:3630–8.
Masuda Y, Kinoshita N, Koumoto K. Morphology control of ZnO crystalline particles in aqueous solution. Electrochim Acta. 2007;53:171–4.
Gao PX, Wang ZL. Nanopropeller arrays of zinc oxide. Appl Phys Lett. 2004;84:2883–5.
Sun XC, Zhang HZ, Xu J, Zhao Q, Wang RM, Yu DP. Shape controllable synthesis of ZnO nanorod arrays via vapor phase growth. Solid State Commun. 2004;129:803–7.
Liu Y, Zhou JE, Larbot A, Persin M. Preparation and characterization of nano-zinc oxide. J Mater Process Technol. 2007;189:379–83.
Raje N, Reddy AVR. Mechanistic aspects of thermal decomposition of thorium oxalate hexahydrate: a review. Thermochim Acta. 2010;505:53–8.
Suino A, Toyama S, Takesue M, Hayashi H, Smith RL Jr. Thermal analysis and mechanism of α-Zn2SiO4:Mn2+ formation from zinc oxalate dihydrate under hydrothermal conditions. Mater Chem Phys. 2013;137:1025–30.
Cong CJ, Hong JH, Liu QY, Liao L, Zhang KL. Synthesis, structure and ferromagnetic properties of Ni-doped ZnO nanparticles. Solid State Commun. 2006;138:511–5.
Peiteado M, Caballero AC, Makovec D. Phase evolution of Zn1−xMnxO system synthesized via oxalate precursors. J Eur Ceram Soc. 2007;27:3915–8.
Małecka B, Drozdz-Ciesla E, Małecki A. Mechanism and kinetics of thermal decomposition of zinc oxalate. Thermochim Acta. 2004;423:13–8.
Majumdar R, Sarkar P, Ray U, Roy MM. Secondary catalytic reactions during thermal decomposition of oxalates of zinc, nickel and iron(II). Thermochim Acta. 1999;335:43–53.
Findorakova L, Svoboda R. Kinetic analysis of the thermal decomposition of Zn(II) 2-chlorobenzoate complex with caffeine. Thermochim Acta. 2012;543:113–7.
Perejon A, Sanchez-Jimenez PE, Criado JM, Perez-Maqueda LA. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B. 2011;115:1780–91.
Svoboda R, Malek J. Applicability of Fraser–Suzuki function in kinetic analysis of complex crystallization processes. J Therm Anal Calorim. 2013;. doi:10.1007/s1097301224459.
Rocco JAFF, Lima JES, Frutuoso AG, Iha K, Ionashiro M, Matos JR, Suárez-Iha MEV. Thermal degradation of a composite solid propellant examined by DSC: kinetic study. J Therm Anal Calorim. 2004;75:551–7.
Liu NA, Fan WC, Dobashi R, Huang LS. Kinetic modeling of thermal decomposition of natural cellulosic materials in air atmosphere. J Anal Appl Pyrolysis. 2002;63:303–25.
Deng CJ, Cai JM, Liu RH. Kinetic analysis of solid-state reactions: evaluation of approximations to temperature integral and their applications. Solid State Sci. 2009;11:1375–9.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2:301–24.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Angermann A, Töpfer J. Synthesis of nanocrystalline Mn–Zn ferrite powders through thermolysis of mixed oxalates. Ceram Int. 2011;37:995–1002.
Behnoudnia F, Dehghani H. Synthesis and characterization of novel three-dimensional-cauliflower-like nanostructure of lead(II) oxalate and its thermal decomposition for preparation of PbO. Inorg Chem Commun. 2012;24:32–9.
Frost RL, Weier ML. Thermal decomposition of humboldtine—a high resolution thermogravimetric and hot stage Raman spectroscopic study. J Therm Anal Calorim. 2004;75:277–91.
Gabal MA, Ata-Allah SS. Concerning the cation distribution in MnFe2O4 synthesized through the thermal decomposition of oxalates. J Phys Chem Solids. 2004;65:995–1003.
Yang L, Wang GZ, Tang CJ, Wang HQ, Zhang LD. Synthesis and photoluminescence of corn-like ZnO nanostructures under solvothermal-assisted heat treatment. Chem Phys Lett. 2005;409:337–41.
Dollimore D. The thermal decomposition of oxalates. A review. Thermochim Acta. 1987;117:331–63.
Wang QF, Wang L, Zhang XW, Mi ZT. Thermal stability and kinetic of decomposition of nitrated HTPB. J Hazard Mater. 2009;172:1659–64.
Salla JM, Morancho JM, Cadenato A, Ramis X. Non-isothermal degradation of a thermoset powder coating in inert and oxidant atmospheres. J Therm Anal Calorim. 2004;72:719–28.
Yi J, Zhao F, Xu S, Zhang L, Gao H, Hu R. Effects of pressure and TEGDN content on decomposition reaction mechanism and kinetics of DB gun propellant containing the mixed ester of TEGDN and NG. J Hazard Mater. 2009;165:853–9.
Sunitha M, Reghunadhan Nair CP, Krishnan K, Ninan KN. Kinetics of Alder-ene reaction of Tris(2-allylphenoxy)triphenoxycyclotriphosphazene and bismaleimides—a DSC study. Thermochim Acta. 2001;374:159–69.
Pan YX, Guan XY, Feng ZY, Li XY, Wu YS. A new method determining mechanism function of solid state reaction—the non-isothermal kinetic of dehydration of nickel(II) oxalate dihydrate in Solid State. Chin J Inorg Chem. 1999;15:247–51.
Gao X, Dollimore D. The thermal decomposition of oxalates. Part 26. A kinetic study of the thermal decomposition of manganese(I1) oxalate dihydrate. Thermochim Acta. 1993;215:47–63.
Turmanoval SCh, Genieva SD, Dimitrova AS, Vlaev LT. Non-isothermal degradation kinetics of filled with rise husk ash polypropene composites. Express Polym Lett. 2008;. doi:10.3144/expresspolymlett.
Noisong P, Danvirutai C. Kinetics and mechanism of thermal dehydration of KMnPO4·H2O in a nitrogen atmosphere. Ind Eng Chem Res. 2010;49:3146–51.
Galwey AK, Brown NE. Thermal decomposition of ionic solids. Netherlands: Elsevier; 1999.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (51272170/21276172) and the Key Programs for Science and Technology Development of Shanxi Province under Contract (No. 20080322035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, C., Mi, J., Shang, S. et al. The study of thermal decomposition kinetics of zinc oxide formation from zinc oxalate dihydrate. J Therm Anal Calorim 115, 1119–1125 (2014). https://doi.org/10.1007/s10973-013-3438-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3438-z