Abstract
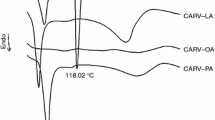
Liposomes and niosomes are known to be efficient vehicles for localized and systemic delivery of particularly lipophilic drugs resulting in their improved bioavailability, targeted delivery, and fewer side effects. These systems consist of bilayered membrane structures comprising amphiphilic molecules like phosphatidylcholine (liposomes) and nonionic surfactants (niosomes). Itraconazole (ITZ) is a widely used insoluble antifungal agent, which is known to be poorly absorbed from available marketed dosage forms. For countering the bioavailability related problem of oral ITZ products, vesicular systems like liposomes and niosomes could provide a rational approach. Drug–excipient interaction is being considered as an essential first step in development of any drug delivery system nowadays. Therefore, the present work describes the evaluation of drug–excipient interactions of ITZ with selected excipients used for development of liposomes and niosomes. Analytical techniques like differential scanning calorimetry, Fourier transform infrared spectroscopy, optical microcopy, and X-ray powder diffraction analysis were utilized for assessing the drug–excipient interactions. Isothermal stress testing was also performed to quantitatively measure the percent change in initial drug content from ITZ–excipient blends kept under stress conditions. The excipients included phospholipids (Phospholipon 90G®, Phospholipon 90H®), surfactants (Span 40 and Span 60), vesicular membrane stabilizer (cholesterol), and a solubilizer (3-hydroxypropyl-betacyclodextrin).






Similar content being viewed by others
References
De Beule K, Van Gestel J. Pharmacology of itraconazole. Drugs. 2001;61(S1):27–37.
Karel DB. Itraconazole: pharmacology, clinical experience and future development. Int J Antimicrob Agents. 1996;6(3):175–81.
Heykants J, Van Peer A, Van de Velde V, Van Rooy P, Meuldermans W, Lavrijsen K, et al. The clinical pharmacokinetics of itraconazole: an overview. Mycoses. 1989;32:67–87. doi:10.1111/j.1439-0507.1989.tb02296.x.
Buchanan CM, Buchanan NL, Edgar KJ, Klein S, Little JL, Ramsey MG, et al. Pharmacokinetics of itraconazole after intravenous and oral dosing of itraconazole-cyclodextrin formulations. J Pharm Sci. 2007;96(11):3100–16. doi:10.1002/jps.20878.
Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172(1–2):33–70. doi:10.1016/S0378-5173(98)00169-0.
Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release. 1994;30(1):1–15. doi:10.1016/0168-3659(94)90039-6.
Kumar N, Shishu, Saini B, Bansal G. Thermal characterization and compatibility studies of itraconazole and excipients for development of solid lipid nanoparticles. J Therm Anal Calorim. 2013. doi:10.1007/s10973-013-3237-6.
Abbas D, Kaloustian J, Orneto C, Piccerelle P, Portugal H, Nicolay A. DSC and physico-chemical properties of a substituted pyridoquinoline and its interaction study with excipients. J Therm Anal Calorim. 2008;93(2):353–60. doi:10.1007/s10973-008-9062-7.
Bruni G, Amici L, Berbenni V, Marini A, Orlandi A. Drug-excipient compatibility studies. Search of interaction indicators. J Therm Anal Calorim. 2002;68(2):561–73. doi:10.1023/a:1016052121973.
Drebushchak VA, Shakhtshneider TP, Apenina SA, Medvedeva AS, Safronova LP, Boldyrev VV. Thermoanalytical investigation of drug-excipient interaction. J Therm Anal Calorim. 2006;86(2):303–9. doi:10.1007/s10973-005-7440-y.
Pani N, Nath L, Acharya S, Bhuniya B. Application of DSC, IST, and FTIR study in the compatibility testing of nateglinide with different pharmaceutical excipients. J Therm Anal Calorim. 2012;108(1):219–26. doi:10.1007/s10973-011-1299-x.
Roumeli E, Tsiapranta A, Pavlidou E, Vourlias G, Kachrimanis K, Bikiaris D, et al. Compatibility study between trandolapril and natural excipients used in solid dosage forms. J Therm Anal Calorim. 2012;111:2109–15. doi:10.1007/s10793-012-2476-2.
Marini A, Berbenni V, Moioli S, Bruni G, Cofrancesco P, Margheritis C, et al. Drug-excipient compatibility studies by physico-chemical techniques; the case of indomethacin. J Therm Anal Calorim. 2003;73(2):529–45. doi:10.1023/a:1025426012578.
Marini A, Berbenni V, Pegoretti M, Bruni G, Cofrancesco P, Sinistri C, et al. Drug-excipient compatibility studies by physico-chemical techniques; the case of atenolol. J Therm Anal Calorim. 2003;73(2):547–61. doi:10.1023/a:1025478129417.
Singh AK, Nath LK. Evaluation of compatibility of tablet excipients and novel synthesized polymer with lamivudine. J Therm Anal Calorim. 2012;108:263–7. doi:10.1007/s10973-011-1650-2.
Six K, Daems T, de Hoon J, Van Hecken A, Depre M, Bouche M-P, et al. Clinical study of solid dispersions of itraconazole prepared by hot-stage extrusion. Eur J Pharm Sci. 2005;24(2–3):179–86. doi:10.1016/j.ejps.2004.10.005.
Koynova R, Caffrey M. Phases and phase transitions of the phosphatidylcholines. Biochim Biophys Acta. 1998;1376:91–145. doi:10.1016/S0304-4157(98)00006-9.
Talegaonkar S, Khan AY, Khar RK, Ahmad FJ, Khan ZI. Development and characterization of paracetamol complexes with hydroxypropyl-β-cyclodextrin. Iranian J Pharm Res. 2007;6:95–9.
Kumar N, Shishu, Bansal G, Kumar S, Jana AK. Preparation and cyclodextrin assisted dissolution rate enhancement of itraconazolium dinitrate salt. Drug Dev Ind Pharm. 2013;39:342–51. doi:10.3109/03639045.2012.681382.
Kumar N, Shishu, Bansal G, Kumar S, Jana AK. Ditosylate salt of itraconazole and dissolution enhancement using cyclodextrins. AAPS PharmSciTech. 2012;. doi:10.1208/s12249-012-9804-5.
Kumar N, Shishu, Kapoor VR. Facile syntheses of novel salts of a triazole antifungal agent with enhanced solubility. J Heterocycl Chem. 2012;. doi:10.1002/jhet.1120.
Silva JPSe, Lobo JMS. Compatibility studies between nebicapone, a novel COMT inhibitor, and excipients using stepwise isothermal high sensitivity DSC method. J Therm Anal Calorim. 2010;102:317–21.
Acknowledgements
This work was supported by a grant provided by University Grants Commission (UGC), New Delhi. The authors are grateful to Nosch Labs (India) for providing itraconazole and Natterman phospholipids (Germany) for providing phospholipids (Phospholipon 90G and Phospholipon 90H) as gift samples.
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, N., Shishu, Bansal, R. et al. Evaluation of compatibility of itraconazole with excipients used to develop vesicular colloidal carriers. J Therm Anal Calorim 115, 2415–2422 (2014). https://doi.org/10.1007/s10973-013-3326-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3326-6