Abstract
Two series of multiblock terpolymers, terpoly(ester-b-ether-b-amide) and terpoly(estersoft-b-ether-b-amide), with the same type of oligoamide (oligolaurolactam (PA12)) hard block and oligoether (oligooxytetramethylene diol soft block were obtained. Oligo (butylene sebacate) was used as oligoester soft block in the first series and oligo (butylene dilinoleate) soft block in the second one. The influence of changes in chemical composition of ester block on the thermal of the terpolymers have been determined by differential scanning calorimetry and dynamic mechanical thermal analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A part of multiblock terpolymers of the –(A x B y C z ) n – type which have the heterophase structure are classified to a group of thermoplastic elastomers (TPE). A macromolecule of the block elastomers consists of flexible and hard blocks distributed alternately. These blocks differ considerably in the physical and chemical properties. The flexible blocks are capable of formation of matrix (soft phase). The hard blocks, as a result of aggregation form the domains of these blocks, constituting the hard phase [1–3].
Such heterophase systems are unique in that the dispersed domain structures are thermodynamically stable in the dispersed state. The phase separation in block copolymers is restricted to molecular dimensions as a consequence of the incompatible block components being joined together, thus preventing gross physical separation of the two components as would occur with their simple mixtures [4, 5].
Two series of multiblock terpolymers—terpoly(ester-b-ether-b-amide) (TEEA) with the same type of oligoamide (oligolaurolactam (PA12)) hard block and oligoether (oligooxytetramethylene diol (PTMO) soft block were obtained. Oligo (butylene sebacinate) was used as oligoester block in the first series and oligo (butylene dilinoleate) soft block in the second one.
The influence of changes in chemical composition of ester block on the structure and thermal and mechanical properties of the terpolymers have been determined by differential scanning calorimetry (DSC) and other standard physical methods.
Subject of examinations
Purpose of paper was to indicate, how a small modification in the chemical structure of only one of the blocks can influence on the thermal, mechanical properties and structure of the multiblock terpolymers. The following terpolymers were selected for such assumed studies:
-
I series: poly(butylene sebacate-b-oxytetramethylene-b-laurolactam) –(BS-b-PTMO-b-PA12) n –,
-
II series: poly(butylene dilinoleate -b-oxytetramethylene-b-laurolactam) –(BDL-b-PTMO-b-PA12) n –.
Two series of multiblock terpolymers, TEEA –(BS-b-PTMO-b-PA12) n – and –(BDL-b-PTMO-b-PA12) n –, with the same type of oligoamide—oligolaurolactam (PA12)—hard block and oligoether—oligooxytetramethylene diol (PTMO)—soft block and different oligoester blocks were obtained.
In the first series, oligo(butylene sebacate) was used as oligoester block (BS) prepared by esterification reaction of sebacic acid (SA) with 1,4-butanediol (BD-1,4) and oligo(butylene dilinoleate)(BDL) block derived from saturated dilinoleic acid (DLA) and 1,4-butanediol (BD-1,4) in the second one.
The influence of changes in chemical composition of ester blocks on the thermal properties of the terpolymers have been determined by DSC and other standard physical methods. Syntheses of the oligoamide PA12 blocks and terpolymers –(BS-b-PTMO-b-PA12) n –, –(BDL-b-PTMO-b-PA12) n – were previously described in details [6, 7].
Methods
The differential calorimetric method was recorded on a DSC-910 (DU Pont Instruments) apparatus. The samples were examined in a triple cycle (heating–cooling–heating) in the temperature range from −100 to +220 °C. The heating and cooling rates were 10 °C min−1.
The dynamical mechanical analysis (DMA) was performed on a DMA Q-800 modulus (TA-Instruments) in the temperature range from −100 to +125 °C at 35 Hz. The storage modulus E′, loss modulus E″, and loss tangent tan δ were determined.
Hardness (H) measurements were performed on a Shore D apparatus (Zwick, type 3100) according to standard DIN 53505 (ISO 863, PN-80/C- 04238).
The limiting viscosity number ([η], LVN) of the poly[ether-b-ester-b-amide] terpolymers in phenol-trichloroethylene (50:50 vol%.) was determined by an Ubbelohde viscometer IIA at 30 °C.
Results and discussion
Description and properties of terpolymers is summarized in Table 1.
The DSC 2nd heating and cooling scans for poly (oligo(butylene sebacate-b-oxytetramethylene-b- laurolactam) -(BS-b-PTMO-b-PA12) n , and poly(butylene dilinoleate-b-oxytetramethylene-b-laurolactam) –(BDL-b-PTMO-b-PA12) n –.are shown in Figs. 1, 2, and the thermal properties of the terpolymers of both series are given in Table 2.
The endotherms connected with melting transition of PA12 (\( T_{{{\text{m}}_{ 3} }} \)) can be observed on DSC curves for first series (Fig. 1), they decrease and flatten along with an increase of degree of polymerization for butylene sebacate.
Crystalline structure of PA12 is disturbed, what can be interpreted as a solution of this block in phase created by other blocks. Similar situation can be observed for melting temperature transition of oligoether segments (\( T_{{{\text{m}}_{ 1} }} \)), which decreases with increasing oligoester content in polymer. For terpolymer with DP BS = 7, melting transition temperatures (\( T_{{{\text{m}}_{ 2} }} \)) appears on thermograms. Glass transition temperature T g increases with increase of soft oligoester molecular weight, which can be connected with their solubility in oligoether phase. Effects of crystallization of oligoamide (\( T_{{{\text{c}}_{ 3} }} \)), oligoester (\( T_{{{\text{c}}_{ 2} }} \)), and oligoether (\( T_{{{\text{c}}_{ 1} }} \)) blocks are presented on cooling curves.
DSC curves of BDL series are present on Fig. 2. For these polymers \( T_{{{\text{m}}_{ 3} }} \) connected with crystalline phase of PA12 is almost constant for DP DLA = 2–7 and for DP DLA = 9 immediately decrease. This phenomenon appears distribution of crystalline phase block PA12 by PTMO which solution in this phase in the same time enthalpy decrease. Confirmation of this disappeared of \( T_{{{\text{m}}_{ 1} }} \) from PTMO. Analogous observations are presented on cooling curves (\( T_{{{\text{c}}_{ 1} }} \), \( T_{{{\text{c}}_{ 3} }} \)). \( T_{{{\text{m}}_{ 2} }} \) related with DLA is not observed because this block is completely amorphous. Glass transition temperature T g decreased with increasing of DP BDL (DLA content), which can indicate miscibility of the both soft blocks (DLA and PTMO). Evidenced by the increase in glass transition range and the associated increase in heat capacity ∆c p.
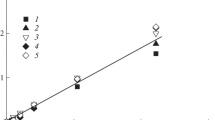
Changes of \( T_{{{\text{m}}_{ 3} }} \) of PA12 for both series are presented on Fig. 3c. For BS series we can observe monotonic decrease with increasing of BS content.
Terpolymers from series based on DLA have higher melting temperatures \( T_{{{\text{m}}_{ 3} }} \) PA12 than series BS but only to DP = 7 and after this value rapidly falls. BS is crystalline and miscibility with PA12 crystalline (from DP = 2–11) in whole range of composition, hence we can observe constant decrease with increasing DP BS. Whereas DLA content promotes crystallization of oligoamide and the above limiting value DP = 7 completely disorders crystalline phase. Melting temperatures related with PTMO presented on Fig. 3b are observed only for terpolymers to DP = 7 for DLA series and to DP = 9 for BS series. For both series \( T_{{{\text{m}}_{ 1} }} \) visibly decreases but for DLA series values are lower. Interesting changes of T g of soft phase are present on Fig. 3a. Temperatures of “pure” substrates are BS is −65 °C, DLA is −68 °C, and PTMO is −90 °C.
T g for BS series increase from −64 to −44 °C because amorphous parts of all components of terpolymers are miscibility. With increasing DP BS relatively decrease PTMO content and components with higher T g start to play a part. For DLA, T g is resultant of amorphous phase (T g from −72 to 85 °C). Almost all PTMO for crystalline phase come into amorphous phase that T g decrease. PTMO is dominant in soft phase [8, 9].
Figures 4, 5 show the results of dynamic mechanical thermal analysis. The relaxation behavior of all samples was studied by DMTA, measurements of the storage modulus E′, the loss modulus E″, and the loss tangent tan δ as a function of the temperature.
Spectra of storage modulus E′ of terpolymers –(BS-b-PTMO-b-PA12) n – (Fig. 4) characterize different temperature ranges, where values of E′ change with the change of the degree of polymerization of BS hard block.
In the temperature range from −100 to −50 °C functions E″ = f (T) have a flat course, the modulus does not change and –(BS-b-PTMO-b-PA12) n – is in the glassy state.
In the interval from −50 to −25 °C modulus decreases, and in the macromolecules there is viscoelastic relaxation processes connected with the glass transition of amorphous phase of the PTMO soft block. This interval moves toward higher temperatures with increasing degree of polymerization of DP BS. The third temperature range is a “flexibility plateau”, where the modulus in this interval is constant. The fourth region observed in the temperature range 0–25 °C is characterized by a further slight decrease of the modulus associated with the melting of the crystalline phase transformation of the PTMO soft block.
With increasing degree of polymerization of the BS block, the width of this range decreases and shifts toward lower temperatures.
For the terpolymer with degree of polymerization DP BS = 11 melting transition of the PTMO soft block is not observed, which was confirmed in the DSC studies.
In the temperature range of 25–50 °C for the terpolymer with a degree of polymerization 7, we can observe a slight decrease of the modulus connected with the melting point of a hard block of BS. The width of this range increases with increasing concentration of BS in terpolymer and is moving toward higher temperatures, which was also confirmed by results of DSC investigation. In the temperature range of 80–130 °C terpolymers achieve the predominance of viscous on elastic properties and their storage modulus is rapidly declining.
Inflection on the curves related to this decrease determine the temperature of polymer softening, and therefore also the upper temperature range of applicability decrease with the increase of terpolymer DP BS. On the curves E″ = f (T) and tan δ = f (T) –(BS-b-PTMO-b-PA12) n –, there is a maximum of α relaxation associated with glass transition temperature of PTMO overlapping with the results of DSC. With the increase of concentration of BS block these peaks flatten and shift toward higher temperatures.
Spectra of storage modulus E′ terpolymers –(BDL-b-PTMO-b-PA12) n – (Fig. 5) in comparison to terpolymers –(BS-b-PTMO-b-PA12) n –, are characterized by lower values, while also we can observe the influence of variable of the degree of polymerization DP BDL on the range of temperature intervals.
In the temperature range of −100 to −70 °C functions E′ = f (T) have a flat course, the modulus does not change and –(BDL-b-PTMO-b-PA12) n – is in the glassy state.
In the interval from −70 to −25 °C a decrease of modulus follows, and in macromolecules viscoelastic relaxation processes occur, which is related with the glass transition of amorphous phase within the PTMO soft block. With increasing degree of polymerization of BDL block, the width of this interval increases and shifts toward lower temperatures. The third temperature range is a “flexibility plateau,” the modulus in this interval is constant. The fourth range observed in the temperature range 0–25 °C is characterized by a further slight decrease of modulus connected with melting transition of the crystalline phase of the PTMO soft block. With increasing degree of polymerization of BDL block, the width of this range decreases and shifts toward lower temperatures. For the terpolymer with a degree of polymerization DP BS = 9 melting transition of PTMO soft block is not observed, which was also confirmed in the DSC results. In the temperature range from −70 to 140 °C terpolymers achieve predominance viscous over elastic properties and their modulus is rapidly decreasing. Inflection on the curves related to this decrease determine the polymer softening temperature, and therefore its upper temperature range of applicability decrease with the increase in terpolymer DP BDL.
On the curves E″ = f (T) and tan δ = f (T) –(BDL-b-PTMO-b-PA12) n – there is maximum of relaxation α associated with PTMO glass transition temperature, overlapping with the results of DSC. With the increase of concentration block BDL these maxima broaden and shift toward lower temperatures. This is a confirmation of test results obtained by DSC, where it was found to increase amorphous phase associated with the miscibility of phases derived from PTMO and DLA.
Conclusions
The results presented in this paper allow to relate the properties of the three component multiblock elastomers with the solubility of one component in the two remaining components of the system.
The terpolymers –(BS-b-PTMO-b-PA12) n – comprise the systems in which the oligo(butylene sebacate (BS) component with DP BS from 2 to 11 dissolves both in the phase of PA12 blocks (hard) and the phase of PTMO blocks (soft). Structure phase in these systems is highly disturbed. Amorphous BS and DLA are miscible with amorphous PTMO while crystalline PTMO are not miscible with crystalline PA12 and crystalline BS is miscible with crystalline PA12.
The terpolymers –(BDL-b-PTMO-b-PA12) n – comprise the systems in which the oligo (BDL) component with DP BS from 2 to 9 are not miscible with crystalline PA12.
Using of two soft blocks in terpolymers deteriorates mechanical properties because of increase of amorphous phase and decay of crystalline phase.
References
Holden G. Thermoplastic elastomers. In: Salamone JC, editor. Polymeric materials encyclopaedia, vol. 11. New York: CRC Press; 1996. p. 8343–53.
Holden G, Legge NR, Quirk R, Schoeder HE. Thermoplastic elastomers. Munich: Hanser Publications; 1996.
Folkes MJ. Processing, structure and properties of block copolymers. London: Elsevier; 1985.
Van Krevelen DW. Properties of polymers, their estimation and correlation with chemical structure. Amsterdam: Elsevier; 1996.
Datta J, Balas A. DSC and thermal stability investigation of novel poly(ester-ether)glycols and poly(ester-ether)urethanes. J Therm Anal Calorim. 2003;74:615–21.
Kozłowska A, Ukielski R, Piątek M. Thermal properties of multiblock thermoplastic elastomers with oligoamide soft blocks derived from dimerized fatty acid. J Therm Anal Calorim. 2006;83:349–53.
Ukielski R, Piątek M. Influence of chemical composition of amide block on the thermal properties and structure of terpolymers. J Therm Anal Calorim. 2004;77:259–67.
Kozłowska A, Majszczyk J, Orłowski M. Relaxation processes in poly(ester-b-amide) thermoplastic elastomer containing poly(butylene sebacate) segments. Rev Adv Mater Sci. 2006;12(2):160–5.
Majszczyk J, Kozłowska A. Thermal, mechanical and dielectric properties of some new poly(amide-block-ester) thermoplastic elastomers. Rev Adv Mater Sci. 2006;12(2):139–44.
Acknowledgements
Financial supports from Polish Ministry for Science and Higher Education research project N N209 216538: “Synthesis and properties of novel ester copolymers for microencapsulation technology” (2010–2013) is acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kozłowska, A., Piątek-Hnat, M. Thermal properties of terpoly(ester-b-ether-b-amide)s with aliphatic ester blocks. J Therm Anal Calorim 111, 977–983 (2013). https://doi.org/10.1007/s10973-012-2396-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2396-1