Abstract
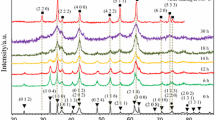
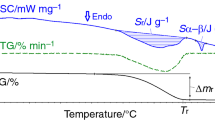
Stoichiometric mixture of CuO and α-Fe2O3 milled in air up to 30 h was subjected to different heat treatments. The evolution of the heat treated milled powders was investigated by X-ray diffraction (XRD). The CuFe2O4 was partially obtained by milling, the material consisting in a mixture of phases. By applying different heat treatments in air and in vacuum, for 2–6 h, in 500–800 °C temperature range the phases composition of the milled samples is changed. A heat treatment at 500 °C in vacuum favours the formation of delafossite (CuFeO2) and tenorite (CuO) phases. If the same heat treatment is made in air, the CuFe2O4 phase formation with a cubic structure is favoured. Differential scanning calorimetry (DSC) investigation realised in Ar atmosphere revealed two large exothermic peaks. The first one is associated with the formation of the delafossite and tenorite phases and the second one with the formation of CuFe2O4. The XRD patterns of the samples subjected to the DSC measurements present maxima corresponding to the delafossite and cuprospinel (CuFe2O4) phases. For the heat treatment at 600 °C in air the phases present in the sample are the same as for the annealing performed at 500 °C: CuFe2O4, α-Fe2O3 and CuO. The heat treatment in air at 800 °C leads to the complete reaction between the different phases and the formation of CuFe2O4 phase in whole the sample volume. The CuFe2O4 ferrite crystallises after this heat treatment in two crystal systems: cubic and tetragonal.





Similar content being viewed by others
References
Cullity BD, Graham CD. Introduction to magnetic materials. 2nd ed. Hoboken: IEEE Press; 2009.
Goldman A. Modern ferrite technology. 2nd ed. Pittsburgh: Springer; 2006.
Tkáčová K, Šepelák V, Šetevulová N, Boldyrev VV. Structure–reactivity study of mechanically activated zinc ferrite. J Solid State Chem. 1996;123:100–8.
Chinnasamy CN, Narayanasamy A, Ponpandian N, Joseyphus RJ, Chattopadhyay K, Shinoda K, Jeyadevan B, Tohji K, Nakatsuka K, Guérault H, Greneche JM. Structure and magnetic properties of nanocrystalline ferrimagnetic CdFe2O4 spinel. Scr Mater. 2001;44:1411–5.
Kodama RH, Berkowitz AE, McNiff EJ Jr, Foner S. Surface spin disorder in NiFe2O4 nanoparticles. Phys Rev Lett. 1996;77(2):394–7.
Goya GF. Handling the particle size and distribution of Fe3O4 nanoparticles through ball milling. Solid State Commun. 2004;130:783–7.
Thapa D, Kulkarni N, Mishra SN, Paulose PL, Ayyub P. Enhanced magnetization in cubic ferrimagnetic CuFe2O4 nanoparticles synthesized from a citrate precursor: the role of Fe2+. J Phys D Appl Phys. 2010;43:195004 (1–5).
Oliver SA, Hamdeh HH, Ho JC. Localized spin canting in partially inverted ZnFe2O4 fine powders. Phys Rev B. 1999;60(5):3400–5.
Srivastava M, Chaubey S, Ojha AK. Investigation on size dependent structural and magnetic behavior of nickel ferrite nanoparticles prepared by sol–gel and hydrothermal methods. Mater Chem Phys. 2009;118:174–80.
Gomes JA, Sousa MH, Tourinho FA. Rietveld structure refinement of the cation distribution in ferrite fine particles studied by X-ray powder diffraction. J Magn Magn Mater. 2005;289:184–7.
Hankare PP, Kadam MR, Patil RP, Garadkar KM, Sasikala R, Tripathi AK. Effect of zinc substitution on structural and magnetic properties of copper ferrite. J Alloys Compd. 2010;501:37–41.
Ding J, McCormick PG, Street R. Formation of spinel Mn-ferrite during mechanical alloying. J Magn Magn Mater. 1997;171:309–14.
Bid S, Pradhan SK. Characterization of crystalline structure of ball-milled nano-Ni–Zn-ferrite by Rietveld method. Mater Chem Phys. 2004;84:291–301.
Verdier T, Nivoix V, Jean M, Hannoyer B. Characterization of nanocrystalline Mn–Zn ferrites obtained by mechanosynthesis. J Mater Sci. 2004;39:5151–4.
Manova E, Tsoncheva T, Paneva D, Popova M, Velinov N, Kunev B, Tenchev K, Mitov I. Nanosized copper ferrite materials: mechanochemical synthesis and characterization. J Solid State Chem. 2011;184:1153–8.
Stewart SJ, Tueros MJ, Cernicchiaro G, Scorzelli RB. Magnetic size growth in nanocrystalline copper ferrite. Solid State Commun. 2004;129:347–51.
Goya GF, Rechenberg HR. Reversibility of the synthesis–decomposition reaction in the ball-milled Cu–Fe–O system. J Phys Condens Matter. 1998;10:11829–40.
Jiang JS, Yang XL, Gao L, Guo JK. Nanostructured CuO–α-Fe2O3 solid solution obtained by high-energy ball milling. Mater Sci Eng A. 2005;392:179–83.
Marinca TF, Chicinaş I, Isnard O. Synthesis, structural and magnetic characterization of nanocrystalline CuFe2O4 as obtained by a combined method reactive milling, heat treatment and ball milling. Ceram Int. 2011. doi:10.1016/j.ceramint.2011.10.026.
Berbenni V, Marini A, Milanese C, Bruni G. Solid state synthesis of CuFe2O4 from Cu(OH)2·CuCO3–4FeC2O4·2H2O mixtures: mechanism of reaction and thermal characterization of CuFe2O4. J Therm Anal Calorim. 2010;99:437–42.
Marinca TF, Chicinaş I, Isnard O, Pop V, Popa F. Synthesis, structural and magnetic characterization of nanocrystalline nickel ferrite-NiFe2O4 obtained by reactive milling. J Alloys Compd. 2011;509:7931–6.
Marinca TF, Chicinaş I, Isnard O, Pop V. Structural and magnetic properties of nanocrystalline ZnFe2O4 powder synthesized by reactive ball milling. Optoelectron Adv Mater Rapid Commun. 2011;5(1–2):39–43.
Hofmann M, Campbell SJ, Kaczmarek WA. Mechanochemical treatment of α-Fe2O3: a neutron diffraction study. Appl Phys A. 2002;74:S1233–5.
Jiang JZ, Goya GF, Rechenberg HR. Magnetic properties of nanostructured CuFe2O4. J Phys Condens Matter. 1999;11:4063–78.
Stewart SJ, Mercader RC, Vandenberghe RE, Cernicchiaro G, Scorzelli RB. Magnetic anomalies and canting effects in nanocrystalline spinel copper ferrites CuxFe3−xO4. J Appl Phys. 2005;97:054304 (1–6).
Deraz NM. Size and crystallinity-dependent magnetic properties of copper ferrite nano-particles. J Alloys Compd. 2010;501:317–25.
Wu X, Zhou K, Wu W, Cui X, Li Y. Magnetic properties of nanocrystalline CuFe2O4 and kinetics of thermal decomposition of precursor. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-2104-6.
Acknowledegements
This study was supported by CNCSIS—UEFISCSU, project number PNII—IDEI code 1519/2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marinca, T.F., Chicinaş, I. & Isnard, O. Influence of the heat treatment conditions on the formation of CuFe2O4 from mechanical milled precursors oxides. J Therm Anal Calorim 110, 301–307 (2012). https://doi.org/10.1007/s10973-012-2250-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2250-5