Abstract
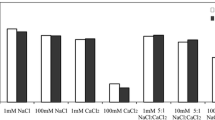
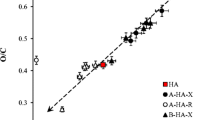
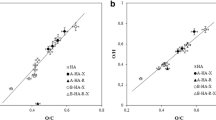
Qualitative and quantitative aspects of hydration of four humic acids (HA) and three fulvic acids (FA) originating from different sources were investigated. DSC experiments at subambient temperatures were carried out in order to monitor differences in ice behavior originating from freezable water surrounding humic molecules. It was found that kinetic effects play a significant role in hydration processes of both HA and FA. In fact, the hydration took part over 21 days which was detected as a progressive decrease in ice melting enthalpy. Simultaneously, the peak shapes and positions changed indicating structural changes in the physical structure of the humic substances. In case of FA, the dependency of melting enthalpy on water concentration showed a linear trend resembling a complete hydration previously observed for water-soluble hydrophilic polymers. In contrast, the melting enthalpy of some HA increased in a step-like way with increasing water content, suggesting preservation of original hydrophobic scaffold during the hydration. The differences between the rather young FA and the rather old HA lead to the conclusion that water can play a significant role in processes of humification. We assume that separation of hydrophobic and hydrophilic domains and thus increase in nanoscale heterogeneity represents an important physical contribution to the overall humification process. It was also demonstrated that the higher content of oxygen in humic molecules is not the only indicator of higher water holding capacity. Instead the porosity of humic matrix seems to contribute as additional parameter into these processes.






Similar content being viewed by others
References
Sutton R, Sposito G. Molecular structure in soil humic substances? The new view. Environ Sci Technol. 2005;39:9009–15.
Stevenson FJ. Humus chemistry: genesis, composition, reactions. 2nd ed. New York: Wiley; 1994.
Schaumann GE, Hurrass J, Műller M, Rotard W. Swelling of organic matter in soil and peat samples: Insights from proton relaxation, water absorption and PAH extraction. In: Ghabbour EA, Davies G, editors. Humic substances: nature’s most versatile materials. New York: Taylor and Francis; 2004. p. 101–17.
Vaxelaire J, Cézac P. Moisture distribution in activated sludges: a review. Water Res. 2004;38:2215–30.
Vesilind PA. The role of water in sludge dewatering. Water Environ Res. 1994;66:4–11.
Tsang KR, Vesilind PA. Moisture distribution in sludges. Water Sci Technol. 1990;22:135–42.
Vesilind PA, Hsu CC. Limits of sludge dewaterability. Water Sci Technol. 1997;36:87–91.
Liu J, Cowman MK. Thermal analysis of semi-dilute hyaluronan solutions. J Therm Anal Cal. 2000;59:547–57.
Prawitwong P, Takigami S, Phillips GO. Phase transition behavior of sorbed water in Konjac mannan. Food Hydrocoll. 2007;21:1368–73.
Hurrass J, Schaumann GE. Hydration kinetics of wettable and water-repellent soils. Soil Sci Soc Am J. 2007;71:280–8.
Schaumann GE. Matrix relaxation and change of water state during hydration of peat. Colloid Surf A. 2005;265:163–70.
Jaeger F, Shchegolikhina A, Van As H, Schaumann GE. Proton NMR relaxometry as a useful tool to evaluate swelling processes of peat soils. Open Magn Reson J. 2010;3:27–45.
Graber ER, Borisover MD. Hydration-facilitated sorption of specifically interacting organic compounds by model soil organic matter. Environ Sci Technol. 1998;32:258–63.
Borisover M, Reddy M, Graber ER. Solvation effect on organic compound interactions in soil organic matter. Environ Sci Technol. 2001;35:2518–24.
Borisover M, Graber ER. Relationship between strength of organic sorbate interactions in NOM and hydration effect on sorption. Environ Sci Technol. 2002;36:4570–7.
Graber ER, Tsechansky L, Borisover M. Hydration-assisted sorption of a probe organic compound at different peat hydration levels: the link solvation model. Environ Sci Technol. 2007;41:547–54.
Schaumann GE, Bertmer M. Do water molecules bridge soil organic matter molecule segments? Eur J Soil Sci. 2008;59:423–9.
Schaumann GE, Leboeuf EJ. Glass transitions in peat: their relevance and the impact of water. Environ Sci Technol. 2005;39:800–6.
Yoshida H, Hakateyama T, Hakateyama H. Characterization of water in polysaccharide hydrogels by DSC. J Therm Anal. 1993;40:483–9.
Takigami S, Takigami M, Phillips GO. Hydration characteristics of the cross-linked hyaluronan derivative hylan. Carbohydr Polym. 1993;22:153–60.
Hakateyama H, Hakateyama T. Interaction between water and hydrophilic polymers. Thermochim Acta. 1998;308:3–22.
Průšová A, Šmejkalová D, Chytil M, Velebný V, Kučerík J. An alternative DSC approach to study hydration of hyaluronan. Carbohydr Polym. 2010;82:498–503.
Kučerík J, Čechlovská H, Bursáková P, Pekař M. The thermodynamic stability and molecular feature of lignite humic acids aggregates studied by high resolution ultrasonic spectroscopy. J Therm Anal Cal. 2009;96:637–43.
Uchida T, Nagayama M, Shibayama T, Gohara K. Morphological investigation of disaccharide molecules for growth inhibition of ice crystals. J Cryst Growth. 2007;299:125–35.
Fernandez JM, Plante AF, Leifeld J, Rasmussen C. Methodological considerations for using thermal analysis in the characterization of soil organic matter. J Therm Anal Cal. 2011;104:289–98.
Siewert C, Demyan MS, Kučerík J. Interrelation between soil respiration and its thermal stability. J Therm Anal Cal. 2010. doi:10.1007/s10973-011-2099-z.
Aquino A, Tunega D, Pasalic H, Schaumann GE. Molecular dynamics simulations of water molecule-bridges in polar domains of humic acids. Environ Sci Technol. 2011. doi:10.1021/es201831g.
Piccolo A. The supramolecular structure of humic substances. Soil Sci. 2001;166:810–32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kučerík, J., Bursáková, P., Průšová, A. et al. Hydration of humic and fulvic acids studied by DSC. J Therm Anal Calorim 110, 451–459 (2012). https://doi.org/10.1007/s10973-011-2178-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2178-1