Abstract
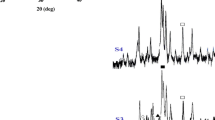
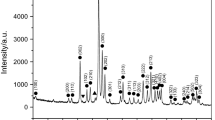
Calcium phosphate biomaterials have long ago attracted the interest of world-wide scientists because they form the main inorganic constituent of the human bones and teeth. Classical approaches to synthesize this ceramic material did not give satisfactory results until present, so new approaches are required. In this article the tricalcium phosphate achievement by a method which is a combination of sol–gel and classic precipitation from solution is presented, starting from CaCl2 as calcium precursor and H3PO4 as phosphorus precursor, without pH adjustment. The reaction mixture was allowed to maturate for 2 months, the time influence on the precipitated material being presented in previous articles. Present studies aimed at the influence of temperature on the structural characteristics of precipitated and maturated material, by means of thermal analysis, X-ray diffraction, infrared spectroscopy, and high-temperature X-ray diffraction. A complex type thermal decomposition takes place while heating the sample to 1000 °C, with superposed and parallel processes. The sample goes through alternative amorphous and crystalline stages before final crystallization of β-tricalcium phosphate takes place. The high-temperature XRD studies offered the great advantage of being both a synthesis and a physico-chemical characterization technique, which along with thermal analysis and infrared spectroscopy, gave a lot of useful information in a very short time.




Similar content being viewed by others
References
Putlyaev VI, Safronova TV. A new generation of calcium phosphate biomaterials: the role of phase and chemical compositions. Glass Ceram. 2006;3–4:99–102.
Jinlong N, Zhenxi Z, Dazong J. Investigation of phase evolution during the thermochemical synthesis of tricalcium phosphate. J Mater Synth Process. 2001;9(5):235–40.
Malysheva AY, Beletskii BI. The state of water in tricalcium phosphate obtained by precipitation from a solution. Glass Ceram. 2001;58(3–4):147–9.
Ioitescu A, Vlase G, Vlase T, Doca N. Kinetics of decomposition of different acid calciul phosphates. J Therm Anal Calorim. 2007;88(1):121–5.
Ioitescu A, Vlase G, Vlase T, Ilia G, Doca N. Synthesis and characterization of hydroxyapatite obtained from different organic precursors by sol-gel method. J Therm Anal Calorim. 2009;96(3):937–42.
Shih W-J, Wang J-W, Wang M-C, Hon M-H. A study on the phase transformation of the nanosized hydroxyapatite synthesized by hydrolysis using in situ high temperature X-ray diffraction. Mater Sci Eng. 2006;C26:1434–8.
Kweh SWK, Khor KA, Cheang P. High temperature in situ XRD of plasma sprayed HA coatings. Biomaterials. 2002;23:381–7.
Bucur A, Bucur R. Time evolution and X-ray diffraction study of a sol-gel calcium phosphate system. Mater Sci Res J. 2010;3(4):1–10.
Bucur A, Bucur R. Border between sol-gel and wet precipitation in calcium phosphates synthesis. In: Morris RE, editor. The sol-gel process: uniformity, polymers and applications. New York: Nova Science Publishers Inc.; 2010. p. 747–56.
Frazier AW, Dillard EF, Waerstad KR, Thrasher RD, Hunter SR, Kohler JJ, Scheib RM. Crystallographic properties of fertilizer compounds, TVA Fertilizer Publ. No y-217, I; 1991.
Guo L, Li H. Phase transformations and structure characterization of calcium polyphosphate during sintering process. J Mater Sci. 2004;39:7041–7.
Reddy BJ, Frost RL, Palmer SJ. A near infrared spectroscopic study of the phosphate mineral pyromorphite Pb5(PO4)3Cl. Spectrochim Acta A. 2008;71(2):430–5.
Stuart Barbara. Infrared spectroscopy: fundamentals and applications. Chichester: John Wiley & Sons Inc.; 2004.
Shiryaev M, Safronova T, Putlyaev V. Calcium phosphate powders synthesized from calcium chloride and potassium hydrophosphate. J Therm Anal Calorim. 2010;101(2):707–13.
Acknowledgements
The authors gratefully thank Dr. Marinela Miclau for all the help provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bucur, A.I., Bucur, R., Vlase, T. et al. Thermal analysis and high-temperature X-ray diffraction of nano-tricalcium phosphate crystallization. J Therm Anal Calorim 107, 249–255 (2012). https://doi.org/10.1007/s10973-011-1753-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1753-9