Abstract
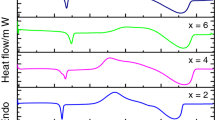
Calorimetric study of Se85−x Te15Sn x (x = 0, 2, 4 and 6) glassy alloys have been performed using Differential Scanning Calorimetry (DSC) under non-isothermal conditions at four different heating rates (5, 10, 15 and 20 °C/min). The glass transition temperature and peak crystallization temperature are found to increase with increasing heating rate. It is remarkable to note that a second glass transition region is associated with second crystallization peak for Sn additive Se–Te investigated samples. Three approaches have been employed to study the glass transition region. The kinetic analysis for the first crystallization peak has been taken by three different methods. The glass transition activation energy, the activation energy of crystallization, and Avrami exponent (n) are found to be composition dependent. The crystallization ability is found to increase with increasing Sn content. From the experimental data, the temperature difference (T p − T g) is found to be maximum for Se83Te15Sn2 alloy, which indicates that this alloy is thermally more stable in the composition range under investigation.





Similar content being viewed by others
References
Khan SA, Zulfequar M, Husain M. On the crystallization kinetics of amorphous Se80In20−x Pb x . Solid State Commun. 2002;123:463–8.
Suri N, Bindra KS, Kumar P, Thangaraj R. Calorimetric studies of Se80−x Te20Bi x bulk samples. J Non-Cryst Solids. 2007;353:1264–7.
Afify N, Hussein MA, El-Kabany N, Fathy N. Structural transformation on Se0.8Te0.2 chalcogenide glass. J Non-Cryst Solids. 2008;354:3260–6.
Chiba R, Funakoshi N. Crystallization of vacuum deposited Te–Se–Cu alloy film. J Non-Cryst Solids. 1988;105:149–54.
Saxena NS. Phase transformation kinetics and related thermodynamic and optical properties in chalcogenide glasses. J Non-Cryst Solids. 2004;345–346:161–8.
Shaaban ER, Kansal I, Shapaan M, Ferreira JMF. Thermal stability and crystallization kinetics of ternary Se–Te–Sb semiconducting glassy alloys. J Therm Anal Calorim. 2009;98:347–54.
Sharma A, Barman PB. Effect of Bi incorporation on the glass transition kinetics of Se85Te15 glassy alloy. J Therm Anal Calorim. 2009;96:413–7.
Kumar H, Mehta N, Singh K. Calorimetric studies of glass transition phenomenon in glassy Se80−x Te20Sn x . Phys Scr. 2009;80:065602.
Kaur G, Komatsu T, Thangaraj R. Crystallization kinetics of bulk amorphous Se–Te–Sn system. J Mater Sci. 2000;35:903–6.
Maharjan NB, Singh K, Saxena NS. Calorimetric studies in Se75Te25−x Sn x chalcogenide glasses. Phys Status Solidi (a). 2003;195:305–10.
Tripathi SK, Sharma V, Thakur A, Sharma J, Saini GSS, Goyal N. Effect of Sb additive on the electrical properties of Se–Te alloy. J Non-Cryst Solids. 2005;351:2468–73.
Othman AA, Amer HH, Osman MA, Dahshan A. Non-isothermal crystallization kinetics study on new amorphous Ga20Sb5S75 and Ga20Sb40S40 chalcogenide glasses. J Non-Cryst Solids. 2005;351:130–5.
Ziani N, Belhadji M, Heireche L, Bouchaour Z, Belbachir M. Crystallization kinetics of Ge20Te80 chalcogenide glasses doped with Sb. Physi B. 2005;358:132–7.
Abu-Sehly AA, Alamri SN, Joraid AA. Measurements of DSC isothermal crystallization kinetics in amorphous selenium bulk samples. J Alloys Compd. 2009;476:348–51.
Ahmad M, Kumar P, Suri N, Kumar J, Thangaraj R. Kinetics of nonisothermal crystallization in Sn10Sb20−x Bi x Se70 glassy semiconductors. Appl Phys A. 2009;94:933–7.
Mehta N, Kumar A. A study of thermal crystallization on glassy Se80Te20 and Se80In20 using DSC technique. J Therm Anal Calorim. 2006;83:401–5.
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Nat Bur Stand. 1956;57:217–21.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Moynihan CT, Easteal AJ, Wilder J, Tucker J. Dependence of the glass transition temperature on heating and cooling rate. J Phys Chem. 1974;78:2673–7.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92.
Gao YQ, Wang W. On the activation energy of crystallization in metallic glasses. J Non-Cryst Solids. 1986;81:129–34.
Addel-Rahim MA, Hafiz MM, Shamekh AM. A study of crystallization kinetics of some Ge–Se–In glasses. Physica B. 2005;369:143–54.
Lafi OA, Imran MMA, Abdullah MK. Glass transition activation energy, glass-forming ability and thermal stability of Se90In10−x Sn x (x = 2, 4, 6 and 8) chalcogenide glasses. Physica B. 2007;395:69–75.
Lucovsky G. Specification of medium range order in amorphous materials. J Non-Cryst Solids. 1987;97:155–8.
Imran MMA, Saxena NS, Husain M. Glass transition phenomena, crystallization kinetics and enthalpy released in binary Se100−x In x (x = 2, 4 and 10) semi conducting glasses. Phys Status Solidi (a). 2000;181:357–68.
Eisenberg A. Glass transition temperatures in amorphous selenium. Polym Lett. 1963;1:177–9.
Lasocka M. The effect of scanning rate on glass transition temperature of splat cooled Te85Ge15. Mater Sci Eng. 1976;23:173–7.
Mahadevan S, Giridhar A, Singh AK. Calorimetric measurements on As–Sb–Se glasses. J Non-Cryst Solids. 1986;88:11–34.
Pratap A, Raval KG, Gupta A, Kulkarni SK. Nucleation and growth of a multicomponent metallic glass. Bull Mater Sci. 2000;23:185–8.
Jain R, Bhandari D, Saxena NS, Sharma SK, Tripathi A. Effect of high-energy heavy ion irradiation on the crystallization kinetics of Co-based metallic glasses. Bull Mater Sci. 2001;24:27–33.
Kauzmann W. The nature of the glassy state and the behavior of liquids at low temperatures. Chem Rev. 1948;43:219–56.
Hruby A. Evaluation of glass-forming tendency by means of DTA. Czech J Phys B. 1972;22:1187–93.
Acknowledgements
This study is financially supported by UGC (Major Research Project), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patial, B.S., Thakur, N. & Tripathi, S.K. Crystallization study of Sn additive Se–Te chalcogenide alloys. J Therm Anal Calorim 106, 845–852 (2011). https://doi.org/10.1007/s10973-011-1579-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1579-5