Abstract
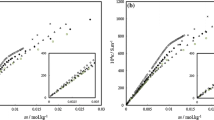
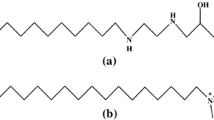
The power–time curves of micellar formation of two anionic surfactants, sodium laurate (SLA) and sodium dodecyl sulfate (SDS), in N,N-dimethyl acetamide (DMA) in the presence of various long-chain alcohols (1-heptanol, 1-octanol, 1-nonanol and 1-decanol) were measured by titration microcalorimetry at 298 K. The critical micelle concentrations (CMCs) of SLA and SDS under various conditions at 298 K were obtained based on the power–time curves. Thermodynamic parameters (\( \Updelta H^\circ_{\text{mic}} \), \( \Updelta S^\circ_{\text{mic}} \) and \( \Updelta G^\circ_{\text{mic}} \)) for micellar systems at 298 K were evaluated according to the power–time curves and the mass action model. The influences of the number of carbon-atom and the concentration of alcohol were investigated. Moreover, combined the thermodynamic parameters at 303, 308 and 313 K in our previous work and those of 298 K in the present work for SLA and SDS in DMA in the presence of long-chain alcohols, an enthalpy–entropy compensation effect was observed. The values of the enthalpy of micellization calculated by direct and indirect methods were made a comparison.

Similar content being viewed by others
References
Baglioni P, Kevan L. Structural effects of alcohol addition to sodium dodecyl sulfate micelles studied by electron spin-echo modulation of 5-doxylstearic acid spin probe. J Phys Chem. 1987;91:1516–8.
Bravo C, Leis JR, Pena ME. Effect of alcohols on catalysis by dodecyl sulfate micelles. J Phys Chem. 1992;96:1957–61.
Forland GM, Sameth J, Hoiland H, Mortensen K. The effect of medium chain length alcohols on the micellar properties of sodium dodecyl sulfate in sodium chloride solutions. J Colloid Interface Sci. 1994;164:163–7.
Pablo ML, Juan MR, Gerardo P, Félix S. Surface tensions, critical micelle concentrations, and standard free energies of micellization of C8−lecithin at different pHs and electrolyte concentrations. J Chem Eng Data. 2002;47:1017–21.
Yogesh R, Sunil SB. CMC determination of an odd carbon chain surfactant (C13E20) mixed with other surfactants using a spectrophotometric technique. J Chem Eng Data. 2006;51:2026–31.
Akhter MS, Al-Alawi SM. The effect of organic additives on critical micelle concentration of non-aqueous micellar solutions. Colloids Surf A. 2000;175:311–20.
Evans DF, Miller DD. In: Friberg SE, Lindman B, editors. Organized solutions. New York: Marcel Dekker; 1992.
Chen D, Zhu JX, Yuan P, Yang SJ, Chen TH, He HP. Preparation and characterization of anion-cation surfactants modified montmorillonite. J Therm Anal Calorim. 2008;94:841–8.
Galán JJ, Del Castillo JL, González-Pérez A, Fuentes-Vázquez V, Rodríguez JR. Solubilization of butanol/pentanol/hexanol in dodecylpyridinium chloride. J Therm Anal Calorim. 2007;87:159–63.
González-Pérez A, Galán JJ, Rodríguez JR. The solubilization of alcohols in micellar solutions. J Therm Anal Calorim. 2003;72:471–9.
Sjoberg M, Henriksson U, Warnheim T. Deuteron nuclear magnetic relaxation of [1,1-2H] hexadecyltrimethylammonium bromide in micellar solutions of nonaqueous polar solvents and their mixtures with water. Langmuir. 1990;6:1205–11.
Beesley AH, Evans DF, Laughlin RG. Evidence for the essential role of hydrogen bonding in promoting amphiphilic self-assembly: measurements in 3-methylsydnone. J Phys Chem. 1988;92:791–3.
Honglin Z, Xiufang Y, Xiangyang L, Haitao S, Yi N, Lili W, et al. Microcalorimetric study of the oscillating extraction system. J Therm Anal Calorim. 2002;68:931–6.
Gucker FT, Pickard HB, Planck RW. A new micro-calorimeter: the heats of dilution of aqueous solutions of sucrose at 20 and 30° and their heat capacities at 25°. J Am Chem Soc. 1939;61:459–70.
Paula S, Süs W, Tuchtenhagen J, Blume A. Thermodynamics of micelle formation as a function of temperature: a high sensitivity titration calorimetry study. J Phys Chem. 1995;99:11742–51.
Dai S, Tam KC. Isothermal titration calorimetric studies of alkyl phenol ethoxylate surfactants in aqueous solutions. Colloids Surf A. 2003;229:157–68.
Yu XF, Wu LL, Zhang HL. Microcalorimetric study on the formation of reversed micelle in P204Li organic phase. Chin J Appl Chem. 2002;3:263–7.
Ralston AW, Eggenberger DW. The effect of organic non-electrolytes upon the conductivities of aqueous solutions of cationic colloidal electrolytes. J Am Chem Soc. 1948;70:983–7.
Li GZ, Lin Y, Guo R, Wang GT. High resolution NMR studies of the solubilised process of micellar solution. Acta Phys Chim Sin. 1986;2:183–9.
Philips JN. The energetic of micelle formation. Trans Faraday Soc. 1955;51:561−9.
Kresheck GC. Water: a comprehensive treatise. New York: Plenum Publications; 1995.
Nusselder JJH, Engberts JB. Toward a better understanding of the driving force for micelle formation and micellar growth. J Colloid Interface Sci. 1992;148:353–61.
Shaw DJ. Introduction to colloid and interface chemistry. 2nd ed. London: Butterworths; 1978.
Shimizu S, Augusto P, Pires R, Seoud AE. Thermodynamics of micellization of benzyl(2-acylaminoethyl)dimethylammonium chloride surfactants in aqueous solutions: a conductivity and titration calorimetry study. Langmuir. 2004;20:9551–9.
Zhang HL, Kong Z, Yan YM, Li GZ, Yu L, Li Z. Studies on the CMC and the thermodynamic function of the anionic surfactants in the DMA/long-chain alcohol systems using a microcalorimetric method. Acta Chim Sin. 2007;65:906–12.
Singh HN, Syed M, Saleem SM, Singh RP, Birdi KS. Micelle formation of ionic surfactants in polar nonaqueous solvents. J Phys Chem. 1980;84:2191–4.
Lumry R, Rafender S. Enthalpy-entropy compensation phenomena in water solutions of proteins and small molecules: a ubiquitous properly of water. Biopolymers. 1970;9:1125–7.
Acknowledgments
The authors are grateful to the Natural Scientific Foundation (Z2007B03) and the Technology Development Project (2006GG2206004) of Shandong Province of China and the Doctoral Fund of the Ministry of Education of China (New Teachers Fund) (070422047) and the Opening Fund of Key Laboratory of Colloid & Interface Chemistry, Ministry of Education, Shandong University of China (200704).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Fei, G., Honglin, Z. et al. Effects of long-chain alcohols on the micellar properties of anionic surfactants in non-aqueous solutions by titration microcalorimetry. J Therm Anal Calorim 96, 859–864 (2009). https://doi.org/10.1007/s10973-009-0059-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0059-7