Abstract
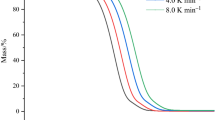
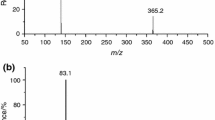
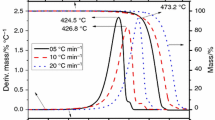
The thermal stability of the ionic liquids (ILs) 1-n-butyl-3-methylimidazolium bromide, [BMIM]Br, and 1-n-octyl-3-methylimidazolium bromide, [OMIM]Br, was evaluated through thermogravimetry (TG). Long-term isothermal TG studies revealed that both of these ILs exhibit appreciable decomposition even at temperatures significantly lower than the onset decomposition temperature, previously determined from fast scan TG experiments. The long-term TG studies of both the ILs showed linear mass loss as a function of time at each temperature of 10 °C interval in the range 533–573 K over a period of 10 h. The kinetics of isothermal decomposition of ILs was analyzed using pseudo-zero-order rate expression. The activation energies for the isothermal decomposition of [BMIM]Br and [OMIM]Br under nitrogen atmosphere are 219.86 and 212.50 kJ mol−1, respectively. The moisture absorption kinetics of these ILs at 25 °C and 30% relative humidity (RH) and at 85 °C and 85% RH were also studied. Water uptake of ILs exposed at 25 °C/30%RH follows a simple saturation behavior in agreement with Weibull model while that at 85 °C/85%RH fortuitously fit into the Henderson–Pabis model.





Similar content being viewed by others
References
Holbrey JD, Seddon KR. Ionic liquids. Clean Prod Processes. 1999;1:223–6.
Seddon KR. Ionic liquids for clean technologies. J Chem Technol Biotech. 1997;68:351–6.
Welton T. Room-temperature ionic liquids: solvents for synthesis and catalysis. Chem Rev. 1999;99:2071–3.
Wilkes JS, Levisky JA, Wilson RA, Charles LH. Dialkylimidazolium chloroaluminate melts: a new class of room-temperature ionic liquids for electrochemistry, spectroscopy, and synthesis. Inorg Chem. 1982;21:1263–4.
Quinn BM, Ding Z, Moulton R, Bard AJ. Novel electrochemical studies of ionic liquids. Langmuir. 2002;18:1734–42.
Olivier-Bourbigou H, Magna L, Morvan D. Ionic liquids and catalysis: recent progress from knowledge to applications. Appl Catal A Gen. 2010;373:1–56.
Hapiot P, Lagrost C. Electrochemical reactivity in room-temperature ionic liquids. Chem Rev. 2008;108:2238–64.
Wang X, Ohlin CA, Lu Q, Fei ZF, Hu J, Dyson PJ. Cytotoxicity of ionic liquids and precursor compounds towards human cell line HeLa. Green Chem. 2007;9:1191–7.
Schneider S, Hawkins T, Rosander M, Vaghjiani G, Chambreau S, Drake G. Ionic liquids as hypergolic fuels. Energy Fuels. 2008;22:2871–2.
Reichardt C. Polarity of ionic liquids determined empirically by means of solvatochromic pyridinium N-phenolate betaine dyes. Green Chem. 2005;7:339–51.
Noda A, Hayamizu K, Watanabe M. Pulsed-gradient spin-echo 1H and 19F NMR ionic diffusion coefficient, viscosity, and ionic conductivity of non-chloroaluminate room-temperature ionic liquids. J Phys Chem B. 2001;105:4603–10.
Martino W, de la Mora JF, Yoshida Y, Saito G, Wilkes J. Surface tension measurements of highly conducting ionic liquids. Green Chem. 2006;8:390–7.
Earle MJ, Esperanca JMSS, Gilea MA, Lopes JNC, Rebelo LPN, Magee JW, Seddon KR, Widegren JA. The distillation and volatility of ionic liquids. Nature. 2006;439:831–4.
Kamavaram V, Reddy RG. Thermal stabilities of di-alkylimidazolium chloride ionic liquids. Int J Therm Sci. 2008;47:773–7.
Kosmulski M, Gustafsson J, Rosenholm JB. Thermal stability of low temperature ionic liquids revisited. Thermochim Acta. 2004;412:47–53.
Baranyai KJ, Deacon GB, MacFarlane DR, Pringle JM, Scott JL. Thermal degradation of ionic liquids at elevated temperatures. Aust J Chem. 2004;57:145–7.
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001;3:156–64.
Seddon KR, Stark A, Torrés MJ. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl Chem. 2000;72:2275–87.
Hanioka S, Maruyama T, Sotani T, Teramoto M, Matsuyama H, Nakashima K, Hanaki M, Kubota F, Goto M. CO2 separation facilitated by task-specific ionic liquids using a supported liquid membrane. J Membr Sci. 2008;314:1–4.
Zhao W, He G, Zhang L, Ju J, Dou H, Nie F, Li C, Liu H. Effect of water in ionic liquid on the separation performance of supported ionic liquid membrane for CO2/N2. J Membr Sci. 2010;350:279–85.
Ramenskaya LM, Grishina EP, Pimenova AM, Gruzdev MS. The influence of water on the physicochemical characteristics of 1-butyl-3-methylimidazolium bromide ionic liquid. Russ J Phys Chem A. 2008;82:1098–103.
Fox DM, Gilman JW, De Long HC, Trulove PC. TG decomposition kinetics of 1-butyl-2, 3-dimethylimidazolium tetrafluoroborate and the thermal effects of contaminants. J Chem Thermodyn. 2005;37:900–5.
Obliosca JM, Arco SD, Huang MH. Synthesis and optical properties of 1-Alkyl-3-methylimidazolium lauryl sulfate ionic liquids. J Fluoresc. 2007;17:613–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arellano, I.H.J., Guarino, J.G., Paredes, F.U. et al. Thermal stability and moisture uptake of 1-alkyl-3-methylimidazolium bromide. J Therm Anal Calorim 103, 725–730 (2011). https://doi.org/10.1007/s10973-010-0992-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0992-5