Abstract
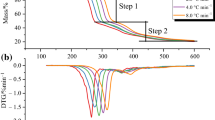
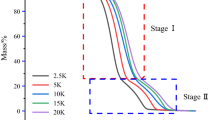
The thermal degradation of two ionic liquids (ILs) was investigated using thermogravimetric analysis (TG) to establish a relationship between the thermokinetics and thermal stability. N-butyl, N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide [BMPyrro][NTf2] and N-octyl, N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide[OMPyrro][NTf2] were subjected to thermogravimetric analysis at varying heating rates of 5, 10 and 20 °C min−1 in the temperature range of 50–600 °C. The data obtained were analysed for thermokinetics using Ozawa, Kissinger and Starink methods using differential thermogravimetric (DTG) techniques, and Flynn–Wall–Ozawa (FWO), Kissinger–Akahira–Sunose (KAS) and Starink methods using TG techniques. The results produced very high regression coefficients (R 2) values around 0.996, which exhibited that they were best fitted by the kinetics equations. The average calculated activation energy (E a) of [BMPyrro][NTf2] using FWO, KAS and Starink methods was 128.6, 123.6 and 124 kJ mol−1, respectively, and 113.7, 107.8 and 108.2 kJ mol−1, respectively, for [OMPyrro][NTf2] using same empirical methods. This emphasizes that the activation energy is strongly related to the length of the side alkyl chain of a given IL. In other words, the longer the side alkyl chain, the lower the activation energy. The E a trends with degree of conversion (α) suggest that a single mechanism without formation of intermediates or short-life intermediates was followed by the pyrolysis kinetics. This study introduced thermokinetics as a tool to study the thermal stability of ionic liquids.
Graphical Abstract







Similar content being viewed by others
References
Maton C, De Vos N, Stevens CV. Ionic liquid thermal stabilities: decomposition mechanisms and analysis tools. Chem Soc Rev. 2013;42:5963–77.
Ignat’ev NV, Finze M, Sprenger JAP, Kerpen C, Bernhardt E, Willner H. New hydrophobic ionic liquids with perfluoroalkyl phosphate and cyanofluoroborate anions. J Fluor Chem. 2015;177:46–54.
Nasir Shah S, Mutalib MIA, Pilus RBM, Lethesh KC. Extraction of naphthenic acid from highly acidic oil using hydroxide-based ionic liquids. Energy Fuels. 2014;29:106–11.
Muhammad N, Gao Y, Khan M, Khan Z, Rahim A, Iqbal F, Khan A, Iqbal J. Effect of ionic liquid on thermo-physical properties of bamboo biomass. Wood Sci Technol. 2015;49(5):897–913.
Ullah Z, Bustam MA, Muhammad N, Man Z, Khan AS. Synthesis and thermophysical properties of hydrogensulfate based acidic ionic liquids. J Solut Chem. 2015;44:875–89.
Ullah Z, Bustam MA, Man Z, Muhammad N, Khan AS. Synthesis, characterization and the effect of temperature on different physicochemical properties of protic ionic liquids. RSC Adv. 2015;5:71449–61.
Xing N, Li Z, Gu C, Pan Y, Guan W. The molar surface Gibbs free energy and its application for aqueous solutions of ionic liquid N-butyl-pyridinium dicyanamide [C4py][DCA]. J Therm Anal Calorim. 2016;126:855–62.
Feng W-Q, Lu Y-H, Chen Y, Lu Y-W, Yang T. Thermal stability of imidazolium-based ionic liquids investigated by TG and FTIR techniques. J Therm Anal Calorim. 2016;125:143–54.
Götz M, Reimert R, Bajohr S, Schnetzer H, Wimberg J, Schubert TJS. Long-term thermal stability of selected ionic liquids in nitrogen and hydrogen atmosphere. Thermochim Acta. 2015;600:82–8.
Salgado J, Parajó JJ, Fernández J, Villanueva M. Long-term thermal stability of some 1-butyl-1-methylpyrrolidinium ionic liquids. J Chem Thermodyn. 2014;74:51–7.
Sronsri C, Noisong P, Danvirutai C. Isoconversional kinetic, mechanism and thermodynamic studies of the thermal decomposition of NH4Co0. 8Zn0. 1Mn0. 1PO4·H2O. J Therm Anal Calorim. 2015;120:1689–701.
Wang G, Ge Z, Luo Y. Thermal decomposition kinetics of poly(3,3′-bisazidomethyl oxetane-3-azidomethyl-3′-methyl oxetane). J Therm Anal Calorim. 2015;122:1515–23.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol JJ. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22:178–83.
Islam MA, Auta M, Kabir G, Hameed B. A thermogravimetric analysis of the combustion kinetics of karanja (Pongamia pinnata) fruit hulls char. Bioresour Technol. 2016;200:335–41.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Blake DM, Moens L, Rudnicki D, Pilath H. Lifetime of imidazolium salts at elevated temperatures. J Sol Energy Eng. 2005;128:54–7.
Burrell AK, Sesto RED, Baker SN, McCleskey TM, Baker GA. The large scale synthesis of pure imidazolium and pyrrolidinium ionic liquids. Green Chem. 2007;9:449–54.
Vyazovkin S. Isoconversional kinetics of thermally stimulated processes. Springer; 2015.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92.
Mu L, Chen J, Yin H, Song X, Li A, Chi X. Pyrolysis behaviors and kinetics of refining and chemicals wastewater, lignite and their blends through TGA. Bioresour Technol. 2015;180:22–31.
Joseph HF, Leo AW. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B: Polym Lett. 1966;4.
Kamavaram V, Reddy RG. Thermal stabilities of di-alkylimidazolium chloride ionic liquids. Int J Therm Sci. 2008;47:773–7.
Kosmulski M, Gustafsson J, Rosenholm JB. Thermal stability of low temperature ionic liquids revisited. Thermochim Acta. 2004;412:47–53.
Salgado J, Villanueva M, Parajó JJ, Fernández J. Long-term thermal stability of five imidazolium ionic liquids. J Chem Thermodyn. 2013;65:184–90.
Taghizadeh MT, Yeganeh N, Rezaei M. Kinetic analysis of the complex process of poly (vinyl alcohol) pyrolysis using a new coupled peak deconvolution method. J Therm Anal Calorim. 2014;118:1733–46.
Irfan Khan M, Azizli K, Sufian S, Man Z, Khan AS. Simultaneous preparation of nano silica and iron oxide from palm oil fuel ash and thermokinetics of template removal. RSC Adv. 2015;5:20788–99.
Ptacek P, Soukal F, Opravil T, Havlica J, Brandstetr J. The kinetic analysis of the thermal decomposition of kaolinite by DTG technique. Powder Technol. 2011;208:20–5.
Tokuda H, Hayamizu K, Ishii K, Susan MABH, Watanabe M. Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J Phys Chem B. 2005;109:6103–10.
Fox DM, Gilman JW, De Long HC, Trulove PC. TGA decomposition kinetics of 1-butyl-2,3-dimethylimidazolium tetrafluoroborate and the thermal effects of contaminants. J Chem Thermodyn. 2005;37:900–5.
Acknowledgements
Authors acknowledge the facilities provided by Centre of Research in Ionic Liquids (CORIL), Research Centre for CO2 Capture (RCCO2C) and Centre for biofuels and biochemical research (CBBR), Universiti Teknologi PETRONAS, Malaysia. The financial support provided by Petroleum Research Fund (PRF), PETRONAS, Malaysia (Cost Centre 0153A3-A30), is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quraishi, K.S., Bustam, M.A., Krishnan, S. et al. Thermokinetics of alkyl methylpyrrolidinium [NTf2] ionic liquids. J Therm Anal Calorim 129, 261–270 (2017). https://doi.org/10.1007/s10973-016-5994-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5994-5