Abstract
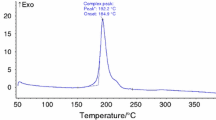
The thermal decomposition behavior of 3,4,5-triamino-1,2,4-triazole dinitramide was measured using a C-500 type Calvet microcalorimeter at four different temperatures under atmospheric pressure. The apparent activation energy and pre-exponential factor of the exothermic decomposition reaction are 165.57 kJ mol−1 and 1018.04 s−1, respectively. The critical temperature of thermal explosion is 431.71 K. The entropy of activation (ΔS ≠), enthalpy of activation (ΔH ≠), and free energy of activation (ΔG ≠) are 97.19 J mol−1 K−1, 161.90 kJ mol−1, and 118.98 kJ mol−1, respectively. The self-accelerating decomposition temperature (T SADT) is 422.28 K. The specific heat capacity of 3,4,5-triamino-1,2,4-triazole dinitramide was determined with a micro-DSC method and a theoretical calculation method. Specific heat capacity (J g−1 K−1) equation is C p = 0.252 + 3.131 × 10−3 T (283.1 K < T < 353.2 K). The molar heat capacity of 3,4,5-triamino-1,2,4-triazole dinitramide is 264.52 J mol−1 K−1 at 298.15 K. The adiabatic time-to-explosion of 3,4,5-triamino-1,2,4-triazole dinitramide is calculated to be a certain value between 123.36 and 128.56 s.

Similar content being viewed by others
References
Arindrajit C, Stefan TT. Confined rapid thermolysis/FTIR/ToF studies of triazolium-based energetic ionic liquids. Thermochim Acta. 2007;466:1–12.
Arindrajit C, Stefan TT, Ping L. Confined rapid thermolysis/FTIR/ToF studies of tetrazolium-based energetic ionic liquids. Thermochim Acta. 2009;485:1–13.
Gerd F, Gerhard H, Thomas MK, Jan JW. A study on the thermal decomposition behavior of derivatives of 1, 5-diamino-1H-tetrazole (DAT): a new of family of energetic heterocyclic-based salts. Thermochim Acta. 2005;437:168–78.
Gregory D, Greg K, Leslie H, Tommy H, Joann L. A new family of energetic ionic liquids 1-amino-3-alkyl-1,2,3-triazolium nitrates. J Chem Crystallogr. 2007;1:15–23.
Huang HF, Meng ZH, Zhou ZM, Gao HX, Zhang J, Wu YK. Energetic salts and energetic ionic liquids. Prog Chem. 2009;1:152–63.
Ou YX, Liu JQ. High energy density compounds. Beijing: National Defence Industry Press; 2005. p. 205–7.
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JJ. Thermal analysis kinetics. 2nd ed. Beijing: Science Press; 2008. (in Chinese).
Yi JH, Zhao FQ, Gao HX, Xu SY, Wang MC, Hu RZ. Preparation, characterization, non-isothermal reaction kinetics, thermodynamic properties, and safety performances of high nitrogen compound: hydrazine 3-nitro-1,2,4-triazol-5-one comple. J Hazard Mater. 2008;153:261–8.
Ma HX, Song JR, Zhao FQ, Hu RZ, Xiao HM. Nonisothermal decomposition kinetics and computational studies on the properties of 2,4,6,8-tetranitro-2,4,6,8-tetraazabicyclo[3,3,1]onan-3,7-dione (TNPDU). J Phys Chem A. 2007;111:8642–9.
Xu SY, Zhao FQ, Yi JH, Hu RZ, Gao HX, Li SW, Hao HX, Pei Q. Thermal behavior and non-isothermal decomposition reaction kinetics of composite modified double base propellant containing Cl-20. Acta Phys-Chim Sin. 2008;24(8):1371–7.
Xue L, Zhao FQ, Xing XL, Gao HX, Yi JH, Hu RZ. Dissolution properties of 1,3,3-trinitroazetidine (TNAZ) in ethyl acetate and N,N-dimethylformamide. Acta Phys-Chim Sin. 2009;25(12):2413–6.
Yi JH, Zhao FQ, Xu SY, Zhang LY, Ren XN, Gao HX, Hu RZ. Effect of pressures on decomposition reaction kinetics of double-base propellant catalyzed with cerium citrate. J Therm Anal Calorim. 2009;92(2):318–23.
Han X, Sun YL, Wang TF, Lin ZK, Li SF, Zhao FQ, Liu ZR, Yi JH, Ren XN. Thermal decomposition of ammonium perchlorate based mixture with fullerenes. J Therm Anal Calorim. 2008;91(2):551–7.
Li JZ, Feng XZ, Hu RZ, Zhao XD, Zhao FQ, Gao HX. Thermal behavior of copper(II) 4-nitroimidazolate. J Therm Anal Calorim. 2009;96(1):195–201.
Zhao FQ, Heng SY, Hu RZ, Gao HX, Han F. A study of kinetic behaviours of effective centralite/stabilizer consumption reaction of propellants using a multi-temperature artificial accelerated ageing test. J Hazard Mater. 2007;145:45–50.
Gao HX, Zhang H, Zhao FQ, Hu RZ, Ma HX, Xu KZ, Xu JH, Gao Y. Kinetic behaviour of the exothermic decomposition reaction of N-guanylurea dinitramide. Acta Phys-Chim Sin. 2008;24(3):453–8.
Xing XL, Xue L, Zhao FQ, Gao HX, Hu RZ. Thermochemical properties of 1,1-diamino-2,2-dinitroethylene (FOX-7) in dimethyl sulfoxide (DMSO). Acta Thermochim. 2009;35:491–6.
Gao HX, Zhao FQ, Hu RZ, Pan Q, Wang BZ, Yang XW, Gao Y, Gao SL, Shi QZ. Thermochemical properties, thermal behavior and decomposition mechanism of 1,1-diamino-2,2-dinitroethylene (DADE). Chin J Chem. 2006;24:177–83.
Xu KZ, Song JR, Zhao FQ, Ma HX, Gao HX, Chang CR, Ren YH, Hu RZ. Thermal behavior, specific heat capacity and adiabatic time to explosion of G(FOX-7). J Hazard Mater. 2008;158:333–37.
Xu KZ, Zhao FQ, Song JR, Ren XL, Gao HX, Xu SY, Hu RZ. Non-isothermal decomposition kinetics of a new high-energy organic potassium salt: K(DNDZ). Bull Korean Chem Soc. 2009;30(10):2259–64.
Xu KZ, Song JR, Zhao FQ, Chang CR, Li M, Wang YY, Hu RZ. Non-isothermal decomposition kinetics, specific heat capacity and adiabatic time-to-explosion of 1-amino-1-hydrazino-2,2-dinitroethylene (AHDNE). J Chin Chem Soc. 2009;27(4):665–71.
Peng JJ, Wu SH, Hou HY, Lin CP, Shu CM. Thermal hazards evaluation of cumene hydroperoxide mixed with its derivatives. J Therm Anal Calorim. 2009;96:783–7.
Hou HY, Liao TS, Duh YS, Shu CM. Thermal hazard studies for dicumyl peroxide by DSC and TAM. J Therm Anal Calorim. 2006;83:167–71.
Zhao FQ, Hu RZ, Zhang H, Gao HX, Zhao HA, Ma HX. A method based on the non-isothermal kinetic equation \( {{{\text{d}}\alpha } \mathord{\left/ {\vphantom {{{\text{d}}\alpha } {{\text{d}}t}}} \right. \kern-\nulldelimiterspace} {{\text{d}}t}} = A_{0} \exp \left( {bT} \right)\left[ {1 + \left( {T - T_{0} } \right)b} \right]f\left( \alpha \right)\; \) to estimate the critical temperature of thermal explosion for energetic materials using non-isothermal DSC. Chem Res Chin Univ. 2009;25:1.
Acknowledgements
Financial support from the National Natural Science Foundation of China (Grant No. 20573098) and the Science and Technology Foundation of the National Defense Key Laboratory of Propellant and Explosive Combustion in China (Grant No. 9140C3501020901).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, L., Zhao, FQ., Xing, XL. et al. Thermal behavior of 3,4,5-triamino-1,2,4-triazole dinitramide. J Therm Anal Calorim 102, 989–992 (2010). https://doi.org/10.1007/s10973-010-0752-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0752-6