Abstract
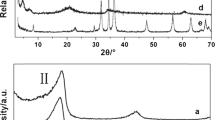
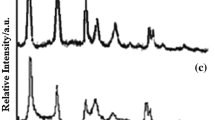
Anionic surfactant and silane modified layered double hydroxides (LDHs) were synthesized through an in situ coprecipitation method. The structure and morphology were characterized by XRD and TEM techniques, and their thermal decomposition processes were investigated using infrared emission spectroscopy (IES) combined with thermogravimetry (TG). The surfactant modified LDHs (H-DS) shows three diffractions located at 1–7° (2θ), while there is only one broad reflection for silane grafted LDHs (H–Si) in this region. The morphologies of the H-DS and H–Si show fibrous exfoliated layers and curved sheets, respectively. The IES spectra and TG curves indicate that alkyl chain combustion and dehydroxylation are overlapped with each other during heating from 373 to 723 K in H-DS and to 873 K in H–Si. Sulfate anion transformation process occurs at 473 K in H-DS and 523 K in H–Si. The derivant of sulfate can exist even above 1073 K. After further decomposition, the metal oxides and the new type of Si–O compounds are formed beginning at around 923 K in silane modified sample.






Similar content being viewed by others
References
Seftel EM, Popovici E, Mertens M, Witte K, Tendeloo G, Cool P, et al. Zn-Al layered double hydroxides: Synthesis, characterization and photocatalytic application. Microporous Mesoporous Mater. 2008;113:296–304.
Goh KH, Lim TT, Dong Z. Application of layered double hydroxides for removal of oxyanions: a review. Water Res. 2008;42:1343–68.
Othman MR, Helwani Z, Fernando WJN. Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: a review. Appl Organomet Chem. 2009;23:335–46.
Fedorov AA, Checkryshkin YS, Rudometova OV, Vnutskikh ZA. Application of inorganic compounds at the thermal processing of polyvinylchloride. Russ J Appl Chem. 2008;81:1673–85.
Angloher S, Kecht J, Bein T. Metal-organic modification of periodic mesoporous silica: multiply bonded systems. Chem Mater. 2007;19:5797–802.
Ingall MDK, Honeyman CH, Mercure JV, Bianconi PA, Kunz RR. Surface functionalization and imaging using monolayers and surface-grafted polymer layers. J Am Chem Soc. 1999;121:3607–13.
Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–30.
Oh S, Kang T, Kim H, Moon J, Hong S, Yi J. Preparation of novel ceramic membranes modified by mesoporous silica with 3-aminopropyltriethoxysilane (APTES) and its application to Cu2+ separation in the aqueous phase. J Membr Sci. 2007;301:118–25.
Yeon YR, Park YJ, Lee JS, Park JW, Kang SG, Jun CH. Sc(OTf)3-mediated silylation of hydroxy functional groups on a solid surface: a catalytic grafting method operating at room temperature. Angew Chem Int Ed. 2008;47:109–12.
Frost RL, Mendelovici E. Modification of fibrous silicates surfaces with organic derivatives: an infrared spectroscopic study. J Colloid Interface Sci. 2006;294:47–52.
Mendelovici E, Frost RL. Pioneer studies on HC1 and silylation treatments of chrysotile. J Colloid Interface Sci. 2005;289:597–9.
Mendelovici E, Frost RL, Kloprogge JT. Modification of chrysotile surface by organosilanes: an IR-photoacoustic spectroscopy study. J Colloid Interface Sci. 2001;238:273–8.
Park AY, Kwon H, Woo AJ, Kim SJ. Layered double hydroxide surface modified with (3-aminopropyl)triethoxysilane by covalent bonding. Adv Mater. 2005;17:106–9.
Wypych F, Bail A, Halma M, Nakagaki S. Immobilization of iron(III) porphyrins on exfoliated MgAl layered double hydroxide, grafted with (3-aminopropyl)triethoxysilane. J Catal. 2005;234:431–7.
Li C, Wang G, Evans DG, Duan X. Incorporation of rare-earth ions in Mg-Al layered double hydroxides: intercalation with an [Eu(EDTA)]− chelate. J Solid State Chem. 2004;177:4569–75.
Kickelbick G. Hybrid materials: synthesis, characterization, and applications. 1st ed. New York: Wiley-VCH; 2007.
Voyer N, Soisnard A, Palmer SJ, Martens WN, Frost RL. Thermal decomposition of the layered double hydroxides of formula Cu6Al2(OH)16CO3 and Zn6Al2(OH)1)CO3. J Therm Anal Calorim. 2009;96:481–5.
Frunza M, Lisa G, Popa MI, Miron ND, Nistor DI. Thermogravimetric analysis of layered double hydroxides with chloramphenicol and salicylate in the interlayer space. J Therm Anal Calorim. 2008;93:373–9.
Palmer SJ, Spratt HJ, Frost RL. Thermal decomposition of hydrotalcites with variable cationic ratios. J Therm Anal Calorim. 2009;95:123–9.
Stanimirova T, Balek V. Characterization of layered double hydroxide Mg-Al-CO3 prepared by re-hydration of Mg-Al mixed oxide. J Therm Anal Calorim. 2008;94:477–81.
Reichle WT. Catalytic reactions by thermally activated, synthetic, anionic clay minerals. J Catal. 1985;94:547–57.
Pesic L, Salipurovic S, Markovic V, Vucelic D, Kagunya W, Jones W. Thermal characteristics of a synthetic hydrotalcite-like material. J Mater Chem. 1992;2:1069–73.
Williams GR, Norquist AJ, O’Hare D. Time-resolved, in situ X-ray diffraction studies of staging during phosphonic acid intercalation into [LiAl2OH)6]Cl·H2O. Chem Mater. 2004;16:975–81.
Williams GR, Dunbar TG, Beer AJ, Fogg AM, O’Hare D. Intercalation chemistry of the novel layered double hydroxides [MAl4(OH)12](NO3)2∙γH2O (M = Zn, Cu, Ni and Co). 1: new organic intercalates and reaction mechanisms. J Mater Chem. 2006;16:1222–30.
Kloprogge JT, Frost RL. Infrared emission spectroscopic study of the thermal transformation of Mg-, Ni- and Co-hydrotalcite catalysts. Appl Catal A-Gen. 1999;184:61–71.
Kloprogge JT, Frost RL. Infrared emission spectroscopy of clay minerals. In: Kloprogge JT, editor. Application of vibrational spectroscopy to clay minerals and layered double hydroxides. Aurora: The Clay Minerals Society; 2005. p. 99–124.
Yang WS, Kim Y, Liu PKT, Sahimi M, Tsotsis TT. A study by in situ techniques of the thermal evolution of the structure of a Mg–Al–CO3 layered double hydroxide. Chem Eng Sci. 2002;57:2945–53.
Tao Q, He HP, Frost RL, Yuan P, Zhu JX. Nanomaterials based upon silylated layered double hydroxides. Appl Surf Sci. 2009;255:4334–40.
Clearfield A, Kieke M, Kwan J, Colon JL, Wang RC. Intercalation of dodecyl sulfate into layered double hydroxides. J Inclusion Phenom Macrocycl. 1991;11:361–78.
Gadsden JA. Infrared spectra of minerals and related inorganic compounds. London: Butterworths; 1975.
Acknowledgements
This is contribution No. IS-1122 from GIGCAS. The financial and infra-structure supports of the National Science Fund for Distinguished Young Scholars (Grant No. 40725006) and Knowledge Innovation Program of the Chinese Academy of Sciences (Grant No. KZCX2-yw-112) are acknowledged. The financial and infra-structure support of the Queensland University of Technology Inorganic Materials Research Program of the School of Physical and Chemical Sciences is gratefully acknowledged. The Australian Research Council (ARC) is thanked for funding. Qi Tao is grateful to The China Scholarship Council for the overseas funding to visit QUT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tao, Q., He, H., Frost, R.L. et al. Thermal decomposition of silylated layered double hydroxides. J Therm Anal Calorim 101, 153–159 (2010). https://doi.org/10.1007/s10973-009-0548-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0548-8