Abstract
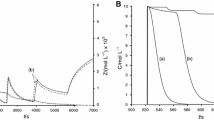
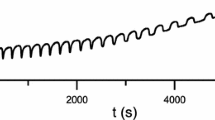
The effect of putrescine (PUT) on KSCN-H2O2-CuSO4-NaOH oscillating system was investigated by calorimetric method. The oscillating reaction was monitored in a closed reactor with stirring, and the result showed that the oscillating period was linearly related with putrescine concentration and the numbers of oscillation decreased with increase in putrescine concentration. When [PUT]=2.83·10−4 M, no oscillation was observed. A possible mechanism is proposed that putrescine is a scavenger of the active-oxygen species. The result of numerical simulation by a simplified mechanism consisting of 18 kinetic steps and 16 variables is consistent with the experimental findings.
Similar content being viewed by others
References
W. C. Bray, J. Am. Chem. Soc., 43 (1921) 1262.
M. Orban and I. R. Epstein, J. Am. Chem. Soc., 107 (1985) 2302.
M. Orban and I. R. Epstein, J. Am. Chem. Soc., 109 (1987) 101.
M. Orban J. Am. Chem. Soc., 108 (1986) 6893.
Qing-Yu Gao, Wan-Hua Xue, Kexue Tongbao, 41 (1996) 1289.
B. Venkataraman and P. G. Sorensen, J. Phys. Chem., 95 (1991) 5707.
E. W. Hansen and P. Ruoff, J. Phys. Chem., 93 (1989) 2696.
J. Ágreda, D. Barrağan and A. Gümez, J. Therm. Anal. Cal., 74 (2003) 875.
R. Chadha, N. Kashid and D. V. S. Jain, J. Therm. Anal. Cal., 81 (2005) 277.
A. A. Saboury, M. S. Atri, M. H. Sanati and M. Sadeghi, J. Therm. Anal. Cal., 83 (2006) 175.
S. Fujisawa and Y. Kadoma, Anticancer Res., 25 (2005) 965.
A. M. L. Kafy, C. G. Haigh and D. A. Lewis, Agents and Actions, 18 (1986) 555.
S. Verma and S. Narayan Mishra, Plant Physiology, 162 (2005) 667.
T. M. Wengenack, G. L. Curran and E. E. Olson, Brain Res., 767 (1997) 128.
Y.-J. Huang, C.-X. Wang and S.-H. Song, J. Wuhan Univ. (Nat. Sci. Ed.), 6 (1994) 76.
R. G. Bates and H. B. Hetzer, J. Phys. Chem., 65 (1961) 667.
R. Jiménez-Prieto, M. Silva and D. Pérez-Bendito, Anal. Chem., 67 (1995) 729.
J.-Z. Gao, H. Yang, X.-H. Liu, Talanta, 57 (2002) 105.
Y. Luo, M. Orban, K. Kustin, J. Am. Chem. Soc., 111 (1989) 4541.
X.-Y. Wang and Q. Zhou, Zhiwuxue Tongbao 19 (2000) 11.
C. W. Gear, Prentice-Hall: Englewood Cliffs, NJ 1971.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jingyan, S., Yuwen, L., Jie, L. et al. Calorimetry studies of a chemical oscillatory system. J Therm Anal Calorim 90, 761–767 (2007). https://doi.org/10.1007/s10973-006-8214-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-8214-x