Abstract
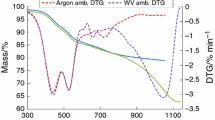
The thermal decomposition behavior of hard coal fly ash (HCA2), obtained from the combustion of an Australian hard coal in thermoelectric power plants, in different atmospheres (air, N2 and N2-H2 mixture), was studied using thermogravimetry (TG), infrared-evolved gas analysis (IR-EGA), differential scanning calorimetry (DSC) and thermodilatometry (DIL) techniques. It was found that changing of the applied atmosphere affects the carbon content of the ash which results in different thermal decomposition behaviors. In air, the carbon content was oxidized to carbon dioxide before the decomposition of carbonate. In N2 or in N2-H2 atmospheres, the carbon content acts as a spacer causing a fewer points of contact between calcium carbonate particles, thus increasing the interface area which results in a decrease of the carbonate decomposition temperature. Following the carbonate decomposition, the iron oxide content of the ash undergoes a reductive decomposition reaction with the unburned carbon. This oxidation-reduction reaction was found to be fast and go to completion in presence of the N2-H2 mixture than in the pure nitrogen atmosphere due to the reducing effect of the hydrogen.
The kinetics of the carbonate decomposition step, in air and N2-H2 mixture was performed under non-isothermal conditions using different integral methods of analysis. The dynamic TG curves obeyed the Avrami-Erofeev equation (A2) in air, and phase boundary controlled reaction equation (R2) in N2-H2 mixture. The change in the reaction mechanism and the difference in the calculated values of activation parameters with the change of the atmosphere were discussed in view of effect of the atmosphere on the carbon content of the ash.
Similar content being viewed by others
References
K. S. Grochawiak, J. Golas, H. Jankowski and S. Kozinski, Fuel, 83 (2004) 1847.
O. E. Manz, Fuel, 78 (1999) 133.
U. M. Graham, Fuel, 76 (1997) 689.
M. J. McCarthy and R. K. Dhir, Fuel, 80 (2001) 1659.
W. Roszczynialski and W. N. Wczelik, J. Therm. Anal. Cal., 77 (2004) 151.
V. Rahhal and R. Talero, J. Therm. Anal. Cal., 87 (2004) 191.
J. Paya, J. Monzo, M. V. Borrachero, E. Perris and F. Amahjour, Cem. Concr. Res., 28 (1998) 675.
P. Fermo, F. Cariati, S. Satacesaria, S. Bruni, M. Lasagni, M. Tettamanti, E. Collina and D. Pitea, Environ. Sci. Technol., 34 (2000) 4370.
M. Fan and R. C. Brown, Energy Fuels, 15 (2001) 15.
G. H. Hemmer, D. Hoff and G. Kasper, Adv. Powder Technol., 14 (2003) 631.
C. Kanaoka, M. Hata and H. Makino, Powder Technol., 118 (2001) 107.
M. Maciejewski, Thermochim. Acta, 355 (2000) 145.
J. H. Khinast, G. F. Krammer, Ch. Brunner and G. Staudinger, Chem. Eng. Sci., 51 (1996) 623.
J. M. Criado, M. Gonzalez, J. Malek and A. Ortega, Thermochim. Acta, 254 (1995) 121.
Y. Wang and W. J. Thomson, Chem. Eng. Sci., 50 (1995) 1373.
J. P. Sanders and P. K. Gallagher, Thermochim. Acta, 388 (2002) 115.
D. Dollimore, P. Tong and K. S. Alexander, Thermochim. Acta, 282/283 (1996) 13.
NIST Chemistry Web Book, Standard reference data base No. 69-March 2003.
El-H. M. Diefallah, Thermochim. Acta, 202 (1992) 1.
El-H. M. Diefallah, A. Y. Obaid, A. H. Qusti, A. A. El-Bellihi and A. M. Abdel Wahab, Thermochim. Acta, 274 (1996) 172.
M. A. Gabal, Thermochim. Acta, 412 (2004) 55.
E. Urbanovici, C. Popescu and E. Segal, J. Therm. Anal. Cal., 58 (1999) 683.
J. H. Flynn, Thermochim. Acta, 282/283 (1996) 35.
C. K. Hsu, Thermochim. Acta, 392-393 (2002) 157.
R. A. Higgins, Engineering Metallurgy, 2nd Ed. Vol. 1, ELBS, London 1974.
T. Hatakeyama and Z. Liu, Handbook of Thermal Analysis, Wiley, Weinheim 1998.
B. V. L’Vov, L. K. Polzik and V. L. Ugolkov, Thermochim. Acta, 390 (2002) 5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabal, M.A., Hoff, D. & Kasper, G. Influence of the atmosphere on the thermal decomposition kinetics of the CaCO3 content of PFBC coal flying ash. J Therm Anal Calorim 89, 109–116 (2007). https://doi.org/10.1007/s10973-005-7494-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7494-x