Abstract
New collagen-based cryogel, isinglass-graphene oxide (IG-GO), was prepared through a simple method. The obtained cryogel was used for rhodamine B (RhB) removal from aqueous environment. The effects of various experimental parameters on the adsorption process, such as pH, contact time, initial dye concentration, adsorbent dosage, and system temperature, were studied. Furthermore, the adsorption isotherms, kinetics and thermodynamics were investigated. The IG-GO cryogel was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR) and nitrogen adsorption/desorption Brunauer-Emmett-Teller (BET) method. The zetta potential results showed that the prepared cryogel possesses a negative charge which make it an appropriate choice for cationic dyes removal. The maximum sorption capacities of the samples were 120 mg/g. The experimental adsorption data are all fitted well with the pseudo-first-order model and Langmuir isotherm. Thermodynamic studies suggest that the adsorption of RhB is highly endothermic in nature. More importantly, the IG-GO cryogel still maintains a relatively high adsorption capacity after five cycles.

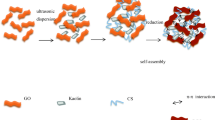
Graphical abstract
Highlights
-
The IG-GO cryogel shows a maximum adsorption capacity of 120 mg/g towards RhB.
-
The reusability of the IG-GO was also evaluated and it was found that the removal efficiency of RhB decreases from 98 to 82% after fifth regeneration.
-
The change in the adsorption behavior was studied through kinetic and isotherm studies.








Similar content being viewed by others
References
Razavi M, Hu S, Thakor AS (2018) A collagen based cryogel bioscaffold coated with nanostructured polydopamine as a platform for mesenchymal stem cell therapy. J Biomed Mater Res Part A 106(8):2213–2228.
He Y et al. (2021) An overview on collagen and gelatin-based cryogels: fabrication, classification, properties and biomedical applications. Polymers 13(14):2299.
Carvalho DN et al. (2020) Marine collagen-chitosan-fucoidan cryogels as cell-laden biocomposites envisaging tissue engineering. Biomed Mater 15(5):055030.
Demir, D, A Vaseashta, and N Bölgen, Macroporous cryogels for water purification, in water safety, security and sustainability. 2021, Springer. 275–290.
Yagub MT et al. (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184.
Goscianska J, Ptaszkowska M, Pietrzak R (2015) Equilibrium and kinetic studies of chromotrope 2R adsorption onto ordered mesoporous carbons modified with lanthanum. Chem Eng J 270:140–149.
Goscianska J, Marciniak M, Pietrzak R (2015) Ordered mesoporous carbons modified with cerium as effective adsorbents for azo dyes removal. Sep Purif Technol 154:236–245.
Zhao D et al. (2013) Adsorption of methyl orange dye onto multiwalled carbon nanotubes. Procedia Environ Sci 18:890–895.
Goscianska J, Pietrzak R (2015) Removal of tartrazine from aqueous solution by carbon nanotubes decorated with silver nanoparticles. Catal Today 249:259–264.
Vučurović VM et al. (2014) Removal of cationic and anionic azo dyes from aqueous solutions by adsorption on maize stem tissue. J Taiwan Inst Chem Eng 45(4):1700–1708.
Yang C et al. (2016) Indium-based metal-organic framework/graphite oxide composite as an efficient adsorbent in the adsorption of rhodamine B from aqueous solution. J Alloy Compd 687:804–812.
Liu H, Ren X, Chen L (2016) Synthesis and characterization of magnetic metal-organic framework for the adsorptive removal of Rhodamine B from aqueous solution. J Ind Eng Chem 34:278–285.
Khamparia S, Jaspal D (2016) Investigation of adsorption of Rhodamine B onto a natural adsorbent Argemone mexicana. J Environ Manag 183:786–793.
Özbaş EE, Öngen A, Gökçe CE (2013) Removal of astrazon red 6B from aqueous solution using waste tea and spent tea bag. Desalin Water Treat 51(40-42):7523–7535.
Su T et al. (2020) Incorporation of dumbbell-shaped and Y-shaped cross-linkers in adjustable pullulan/polydopamine hydrogels for selective adsorption of cationic dyes. Environ Res 182:109010.
de Luna MS et al. (2019) Optimization of dye adsorption capacity and mechanical strength of chitosan aerogels through crosslinking strategy and graphene oxide addition. Carbohydr Polym 211:195–203.
Majewska-Nowak K, Winnicki T, Wiśniewski J (1989) Effect of flow conditions on ultrafiltration efficiency of dye solutions and textile effluents. Desalination 71(2):127–135.
Sun D et al. (2010) Adsorption of anionic dyes from aqueous solution on fly ash. J Hazard Mater 181(1-3):335–342. p
Muthukumar M et al. (2004) Optimisation of ozone treatment for colour and COD removal of acid dye effluent using central composite design experiment. Dyes Pigments 63(2):127–134.
Kotsmar C et al. (2010) Equilibrium and dynamics of adsorption of mixed β-casein/surfactant solutions at the water/hexane interface. Colloids Surf A: Physicochemical Eng Asp 354(1-3):210–217.
Lupa L et al. (2015) Ionic liquids impregnated onto inorganic support used for thallium adsorption from aqueous solutions. Sep Purif Technol 155:75–82.
Chakraborty, R, et al., Adsorption of heavy metal ions by various low-cost adsorbents: a review. Int J Environ Anal Chem, 2020: p. 1–38.
Mende M et al. (2016) Simultaneous adsorption of heavy metal ions and anions from aqueous solutions on chitosan—Investigated by spectrophotometry and SEM-EDX analysis. Colloids Surf A: Physicochem Eng Asp 510:275–282.
Ashokkumar M, Ajayan PM (2021) Materials science perspective of multifunctional materials derived from collagen. Int Mater Rev 66(3):160–187.
Dolatkhah Z, Javanshir S, Bazgir A (2019) Isinglass–palladium as collagen peptide–metal complex: a highly efficient heterogeneous biocatalyst for Suzuki cross-coupling reaction in water. J Iran Chem Soc 16(7):1473–1481.
Dolatkhah Z et al. (2018) Magnetic Isinglass a nano‐bio support for copper immobilization: Cu–IG@ Fe3O4 a heterogeneous catalyst for triazoles synthesis. ChemistrySelect 3(19):5486–5493.
Javanshir S et al. (2017) Caspian Isinglass, a versatile and sustainable biocatalyst for domino synthesis of spirooxindoles and spiroacenaphthylenes in water. Monatshefte für Chem-Chem Monthly 148(4):703–710.
Hummers Jr WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339.
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186(1):458–465.
Chaudhuri B et al. (2015) Myoblast differentiation of human mesenchymal stem cells on graphene oxide and electrospun graphene oxide–polymer composite fibrous meshes: importance of graphene oxide conductivity and dielectric constant on their biocompatibility. Biofabrication 7(1):015009.
Davidenko N et al. (2010) Collagen–hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomaterialia 6(10):3957–3968.
Shin YC et al. (2015) Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. J Nanobiotechnol 13(1):1–11.
Deepachitra R, Ramnath V, Sastry T (2014) Graphene oxide incorporated collagen–fibrin biofilm as a wound dressing material. RSC Adv 4(107):62717–62727.
Singh P et al. (2011) Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem 124(1):97–105.
Pourian E et al. (2018) Ultrasonic-assisted preparation, characterization, and use of novel biocompatible core/shell Fe3O4@ GA@ isinglass in the synthesis of 1, 4-dihydropyridine and 4 H-pyran derivatives. ACS Omega 3(5):5012–5020.
Schlumberger C, Thommes M (2021) Characterization of hierarchically ordered porous materials by physisorption and mercury porosimetry—a tutorial review. Adv Mater Interfaces 8(4):2002181.
Senthilkumaar S et al. (2011) Adsorption behavior of organic dyes in biopolymers impregnated with H3PO4: Thermodynamic and equilibrium studies. Chem Eng Technol 34(9):1459–1467.
Cheng Z-L, Li Y-x, Liu Z (2018) Study on adsorption of rhodamine B onto Beta zeolites by tuning SiO2/Al2O3 ratio. Ecotoxicol Environ Saf 148:585–592.
Ptaszkowska-Koniarz M, Goscianska J, Pietrzak R (2018) Removal of rhodamine B from water by modified carbon xerogels. Colloids Surf A: Physicochem Eng Asp 543:109–117.
Hou M-F et al. (2011) Removal of rhodamine B using iron-pillared bentonite. J Hazard Mater 186(2-3):1118–1123.
Das SK et al. (2006) Adsorption behavior of rhodamine B on rhizopus o ryzae biomass. Langmuir 22(17):7265–7272
Wang X et al. (2018) Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 206:587–596
Kuo C-Y, Wu C-H, Wu J-Y (2008) Adsorption of direct dyes from aqueous solutions by carbon nanotubes: Determination of equilibrium, kinetics and thermodynamics parameters. J Colloid Interface Sci 327(2):308–315
Al-Ghouti MA et al. (2009) Adsorption behaviour of methylene blue onto Jordanian diatomite: a kinetic study. J Hazard Mater 165(1–3):589–598
Sierra-Trejo PV, Guibal E, Louvier-Hernández JF (2020) Arsenic sorption on chitosan-based sorbents: Comparison of the effect of molybdate and tungstate loading on As (V) sorption properties. J Polym Environ 28(3):934–947.
Tang L et al. (2014) Cobalt nanoparticles-embedded magnetic ordered mesoporous carbon for highly effective adsorption of rhodamine B. Appl Surf Sci 314:746–753.
El Haddad M et al. (2016) Evaluation of performance of animal bone meal as a new low-cost adsorbent for the removal of a cationic dye Rhodamine B from aqueous solutions. J Saudi Chem Soc 20:S53–S59.
Mohammadi M et al. (2010) Removal of rhodamine B from aqueous solution using palm shell-based activated carbon: adsorption and kinetic studies. J Chem Eng Data 55(12):5777–5785.
Zou J et al. (2013) Structure and adsorption properties of sewage sludge-derived carbon with removal of inorganic impurities and high porosity. Bioresour Technol 142:209–217.
da Silva Lacerda V et al. (2015) Rhodamine B removal with activated carbons obtained from lignocellulosic waste. J Environ Manag 155:67–76.
Banerjee D et al. (2017) Pseudo first ordered adsorption of noxious textile dyes by low-temperature synthesized amorphous carbon nanotubes. Phys E: Low-dimensional Syst Nanostruct 87:68–76.
Xie Y et al. (2017) Fibrous N-doped hierarchical porous carbon microspheres: Synthesis and adsorption performance. Chem Eng J 323:224–232.
Kim T-S et al. (2018) Fast adsorption kinetics of highly dispersed ultrafine nickel/carbon nanoparticles for organic dye removal. Appl Surf Sci 439:364–370.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SA, ME, and SJ. The first draft of the manuscript was written by SA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azadi, S., Esmkhani, M. & Javanshir, S. New collagen-based cryogel as bio-sorbent materials for Rhodamine B removal from aqueous environments. J Sol-Gel Sci Technol 103, 405–415 (2022). https://doi.org/10.1007/s10971-022-05839-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05839-4