Abstract
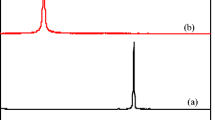
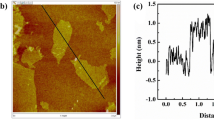
The assembly of graphene oxide with biomass or polymers to form 3D hydrogels with excellent mechanical properties has become a research hotspot. In this work, the sponge-like kaolin/chitosan (CS)/reduced graphene oxide (rGO) composite was prepared for adsorption by simple self-assembly without cross-linking agent. The morphology, composition, surface properties, and pore size of as-prepared materials were characterized by Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), zeta potential analyzer, Brunauer-Emmett-Teller surface area measurement (BET), and scanning electron microscopy (SEM). The effects of raw material ratio, contact time, temperature, pH, ionic strength, and recycling times on adsorption performance were investigated in detail. The results indicate that the composite has good absorption capacity for Cr(VI) and alizarin yellow R (AYR). Besides, composite hydrogel also exhibits excellent flexibility and good repeatability, which confirms its great potential as an adsorbent to remove pollutants in the water environment.










Similar content being viewed by others
References
Boukhemkhem A, Rida K (2017) Improvement adsorption capacity of methylene blue onto modified Tamazert kaolin. Adsorpt Sci Technol 35:753–773
Cao N, Lyu Q, Li J, Wang Y, Yang B, Szunerits S, Boukherroub R (2017) Facile synthesis of fluorinated polydopamine/chitosan/reduced graphene oxide composite aerogel for efficient oil/water separation. Chem Eng J 326:17–28
Chávez-Delgado ME, Kishi-Sutto CV, Albores de la-Riva XN, Rosales-Cortes M, Gamboa-Sánchez P (2014) Topic usage of kaolin-impregnated gauze as a hemostatic in tonsillectomy. J Surg Res 192:678–685
Crini G, Badot P-M (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
Fan L, Luo C, Li X, Lu F, Qiu H, Sun M (2012a) Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J Hazard Mater 215-216:272–279
Fan L, Zhang Y, Li X, Luo C, Lu F, Qiu H (2012b) Removal of alizarin red from water environment using magnetic chitosan with Alizarin Red as imprinted molecules. Colloid Surface B 91:250–257
Felfel RM, Gideon-Adeniyi MJ, Zakir Hossain KM, Roberts GAF, Grant DM (2019) Structural, mechanical and swelling characteristics of 3D scaffolds from chitosan-agarose blends. Carbohydr Polym 204:59–67
Feng X, Liang C, Yu J, Jiang X (2018) Facile fabrication of graphene oxide-polyethylenimine composite and its application for the Cr(VI) removal. Sep Sci Technol 262:597–606
Feng Y, Jiao T, Yin J, Zhang L, Zhang L, Zhou J, Peng Q (2019) Facile preparation of carbon nanotube-Cu2O nanocomposites as new catalyst materials for reduction of p-nitrophenol. Nanoscale Res Lett 14:78
Gao H, Du J, Liao Y (2019) Removal of chromium(VI) and orange II from aqueous solution using magnetic polyetherimide/sugarcane bagasse. Cellulose 26:3285–3297
Guo R, Wang R, Yin J, Jiao T, Huang H, Zhao X, Zhang L, Li Q, Zhou J, Peng Q (2019) Fabrication and highly efficient dye removal characterization of beta-cyclodextrin-based composite polymer fibers by electrospinning. Nanomaterials-Basel 9:127
Hu H, Zhao Z, Wan W, Gogotsi Y, Qiu J (2013) Ultralight and highly compressible graphene aerogels. Adv Mater 25:2219–2223
Huang Y, Zeng M, Feng Z, Yin D, Xu Q, Fan L (2016) Graphene oxide-based composite hydrogels with self-assembled macroporous structures. RSC Adv 6:3561–3570
Huang X, Wang R, Jiao T, Zou G, Zhan F, Yin J, Zhang L, Zhou J, Peng Q (2019) Facile preparation of hierarchical AgNP-loaded MXene/Fe3O4/polymer nanocomposites by electrospinning with enhanced catalytic performance for wastewater treatment. ACS Omega 4:1897–1906
Iqbal MJ, Ashiq MN (2007) Adsorption of dyes from aqueous solutions on activated charcoal. J Hazard Mater 139:57–66
Jiang X, Luo H, Yin Y, Zhou W (2017) Facile synthesis of MoS2/reduced graphene oxide composites for efficient removal of Cr(VI) from aqueous solutions. RSC Adv 7:24149–24156
Kong Q, Wei J, Hu Y, Wei C (2019) Fabrication of terminal amino hyperbranched polymer modified graphene oxide and its prominent adsorption performance towards Cr(VI). J Hazard Mater 363:161–169
Kyzas GZ, Bikiaris DN, Seredych M, Bandosz TJ, Deliyanni EA (2014) Removal of dorzolamide from biomedical wastewaters with adsorption onto graphite oxide/poly(acrylic acid) grafted chitosan nanocomposite. Bioresour Technol 152:399–406
Li JL, Kudin KN, McAllister MJ, Prud’homme RK, Aksay IA, Car R (2006) Oxygen-driven unzipping of graphitic materials. Phys Rev Lett 96:176101 (1-4)
Liang C, Feng X, Yu J, Jiang X (2018a) Facile one-step hydrothermal syntheses of graphene oxide–MnO2 composite and their application in removing heavy metal ions. Micro Nano Lett 13:1179–1184
Liang Y, Xu C, Li G, Liu T, Liang JF, Wang X (2018b) Graphene-kaolin composite sponge for rapid and riskless hemostasis. Colloid Surface B 169:168–175
Lim JY, Mubarak NM, Abdullah EC, Nizamuddin S, Khalid M, Inamuddin (2018) Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals — a review. J Ind Eng Chem 66:29–44
Liu L, Liu S, Zhang Q, Li C, Bao C, Liu X, Xiao P (2012) Adsorption of Au(III), Pd(II), and Pt(IV) from aqueous solution onto graphene oxide. J Chem Eng Data 58:209–216
Minh TD, Lee BK, Nguyen-Le MT (2018) Methanol-dispersed of ternary Fe3O4@gamma-APS/graphene oxide-based nanohybrid for novel removal of benzotriazole from aqueous solution. J Environ Manag 209:452–461
Mouni L, Belkhiri L, Bollinger J-C, Bouzaza A, Assadi A, Tirri A, Dahmoune F, Madani K, Remini H (2018) Removal of methylene blue from aqueous solutions by adsorption on kaolin: kinetic and equilibrium studies. Appl Clay Sci 153:38–45
Paulista Neto AJ, Fileti EE (2018) Elucidating the amphiphilic character of graphene oxide. Phys Chem Chem Phys 20:9507–9515
Sharma P, Hussain N, Borah DJ, Das MR (2013) Kinetics and adsorption behavior of the methyl blue at the graphene oxide/reduced graphene oxide nanosheet–water interface: a comparative study. J Chem Eng Data 58:3477–3488
Sherlala AIA, Raman AAA, Bello MM, Asghar A (2018) A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 193:1004–1017
Shi H, Li W, Zhong L, Xu C (2014) Methylene blue adsorption from aqueous solution by magnetic cellulose/graphene oxide composite: equilibrium, kinetics, and thermodynamics. Ind Eng Chem Res 53:1108–1118
Wang H, Yuan X, Wu Y, Chen X, Leng L, Wang H, Li H, Zeng G (2015) Facile synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic composites and its application for the Cr(VI) removal. Chem Eng J 262:597–606
Wang C, Yin J, Wang R, Jiao T, Huang H, Zhou J, Zhang L, Peng Q (2019a) Facile preparation of self-assembled polydopamine-modified electrospun fibers for highly effective removal of organic dyes. Nanomaterials-Basel 9:116
Wang XL, Guo DM, An QD, Xiao ZY, Zhai SR (2019b) High-efficacy adsorption of Cr(VI) and anionic dyes onto beta-cyclodextrin/chitosan/hexamethylenetetramine aerogel beads with task-specific, integrated components. Int J Biol Macromol 128:268–278
Wu Z (2004) Organic dye adsorption on mesoporous hybrid gels. Chem Eng J 102:277–282
Wu Z, Zhong H, Yuan X, Wang H, Wang L, Chen X, Zeng G, Wu Y (2014) Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res 67:330–342
Wu K, Yu J, Jiang X (2017) Multi-walled carbon nanotubes modified by polyaniline for the removal of alizarin yellow R from aqueous solutions. Adsorpt Sci Technol 36:198–214
Xu X, Jiang X-Y, Jiao F-P, Chen X-Q, Yu J-G (2018) Tunable assembly of porous three-dimensional graphene oxide-corn zein composites with strong mechanical properties for adsorption of rare earth elements. J Taiwan Inst Chem Eng 85:106–114
Yang J-Y, Jiang X-Y, Jiao F-P, Yu J-G (2018) The oxygen-rich pentaerythritol modified multi-walled carbon nanotube as an efficient adsorbent for aqueous removal of alizarin yellow R and alizarin red S. Appl Surf Sci 436:198–206
Yu Z, Zhang X, Huang Y (2013) Magnetic chitosan–Iron(III) hydrogel as a fast and reusable adsorbent for chromium(VI) removal. Ind Eng Chem Res 52:11956–11966
Yu P, Bao R-Y, Shi X-J, Yang W, Yang M-B (2017a) Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr Polym 155:507–515
Yu P, Wang H-Q, Bao R-Y, Liu Z, Yang W, Xie B-H, Yang M-B (2017b) Self-assembled sponge-like chitosan/reduced graphene oxide/montmorillonite composite hydrogels without cross-linking of chitosan for effective Cr(VI) sorption. ACS Sustain Chem Eng 5:1557–1566
Zhang Y, Liu Y, Wang X, Sun Z, Ma J, Wu T, Xing F, Gao J (2014) Porous graphene oxide/carboxymethyl cellulose monoliths, with high metal ion adsorption. Carbohydr Polym 101:392–400
Zhang J, Chen S, Zhang H, Wang X (2017a) Removal behaviors and mechanisms of hexavalent chromium from aqueous solution by cephalosporin residue and derived chars. Bioresour Technol 238:484–491
Zhang Q, Yang Q, Phanlavong P, Li Y, Wang Z, Jiao T, Peng Q (2017b) Highly efficient lead(II) sequestration using size-controllable polydopamine microspheres with superior application capability and rapid capture. ACS Sustain Chem Eng 5:4161–4170
Zhang Q, Bolisetty S, Cao Y, Handschin S, Adamcik J, Peng Q, Mezzenga R (2019a) Selective and efficient removal of fluoride from water: in situ engineered amyloid fibril/ZrO2 hybrid membranes. Angew Chem 58:6012–6016
Zhang Y, Luo K, Yu J, Jiang X (2019b) Green fabrication of SH-modified GO composite for heavy metal ions removal in aqueous solution. Micro Nano Lett 14:373–378
Zhao H, Jiao T, Zhang L, Zhou J, Zhang Q, Peng Q, Yan X (2015) Preparation and adsorption capacity evaluation of graphene oxide-chitosan composite hydrogels. Science Chian Materials 58:811–818
Zhao X-R, Xu X, Teng J, Zhou N, Zhou Z, Jiang X-Y, Jiao F-P, Yu J-G (2019) Three-dimensional porous graphene oxide-maize amylopectin composites with controllable pore-sizes and good adsorption-desorption properties: facile fabrication and reutilization, and the adsorption mechanism. Ecotoxicol Environ Saf 176:11–19
Zhu HY, Jiang R, Xiao L, Zeng GM (2010) Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized gamma-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Bioresour Technol 101:5063–5069
Funding
This work was supported by the National Natural Science Foundation of China (No. 21571191 and No. 51674292) and the Key Laboratory of Hunan Province for Water Environment and Agriculture Product Safety (2018TP1003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1082 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Chen, S., Feng, X. et al. Self-assembly of sponge-like kaolin/chitosan/reduced graphene oxide composite hydrogels for adsorption of Cr(VI) and AYR. Environ Sci Pollut Res 26, 28898–28908 (2019). https://doi.org/10.1007/s11356-019-06068-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06068-z