Abstract
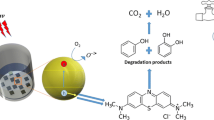
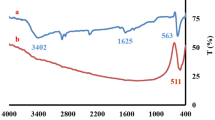
The present work reports the synthesis of CeO2 nanoparticles (NPs) via chemical route by optimizing synthesis parameters such as solvent, capping agent, and calcination temperature. The prepared samples were characterized via X-ray diffraction (XRD), transmission electron microscopy, Fourier-transformed infrared spectroscopy, Raman spectroscopy, and UV-visible spectroscopy for structural, morphological, and optical studies. The XRD results confirmed the formation of cubic fluorite structured CeO2 with an average crystallite size ~5–11 nm. The enhancement in crystallite size was observed along with agglomerated irregular spherical-shaped NPs after calcination at higher temperatures. The use of capping agent (thioglycerol; TG) led to the reduced aggregation of NPs. With the help of UV-visible spectroscopy, a redshift was observed in the maximum absorbance peak as an effect of calcination. The NPs synthesized in ethanol as solvent exhibited better degradation efficiency for crystal violet dye than that synthesized in water medium due to high surface to volume ratio and surface functionalities inducing more active sites for the creation of electrons hole pair. It was observed that (TG) capped samples exhibited more photocatalytic activity toward the degradation of crystal violet dye than uncapped samples due to less agglomeration of particles. Furthermore, in the presence of the optimum amount of H2O2 the catalytic activity was achieved up to 97% within 90 min only.

Highlights
-
Co-precipitation synthesis of ceria nanoparticles (CeO2 NPs) via varying calcination temperature, solvent medium, and capping agent.
-
Photocatalytic degradation of crystal violet (CV) dye under UV illumination has been studied.
-
Significant roles of surface area, hydroxyl groups, and oxygen vacancies in catalytic performance of CeO2 NPs are discussed.
-
Capped CeO2 synthesized in ethanol medium exhibited enhanced photocatalytic activity.














Similar content being viewed by others
References
Long S, Zhao L, Shi T et al. (2018) Pollution control and cost analysis of wastewater treatment at industrial parks in Taihu and Haihe water basins, China. J Clean Prod 172:2435–2442. https://doi.org/10.1016/j.jclepro.2017.11.167
Evans AE, Mateo-Sagasta J, Qadir M et al. (2019) Agricultural water pollution: key knowledge gaps and research needs. Curr Opin Environ Sustain 36:20–27. https://doi.org/10.1016/j.cosust.2018.10.003
Channei D, Inceesungvorn B, Wetchakun N, et al. (2014) Photocatalytic degradation of methyl orange by CeO2 and Fe-doped CeO2 films under visible light irradiation. Sci Rep 4. https://doi.org/10.1038/srep05757
Ibhadon AO, Fitzpatrick P (2013) Heterogeneous photocatalysis: Recent advances and applications. Catalysts 3:189–218. https://doi.org/10.3390/catal3010189
Mittal M, Sharma M, Pandey OP (2016) Fast and quick degradation properties of doped and capped ZnO nanoparticles under UV-Visible light radiations. Sol Energy 125:51–64. https://doi.org/10.1016/j.solener.2015.12.003
Choudhary S, Sahu K, Bisht A et al. (2020) Template-free and surfactant-free synthesis of CeO2 nanodiscs with enhanced photocatalytic activity. Appl Surf Sci 503:144102. https://doi.org/10.1016/j.apsusc.2019.144102
Ranjith KS, Dong CL, Lu YR et al. (2018) Evolution of visible photocatalytic properties of Cu-doped CeO2 nanoparticles: role of Cu2+-mediated oxygen vacancies and the mixed-valence states of Ce ions. ACS Sustain Chem Eng 6:8536–8546. https://doi.org/10.1021/acssuschemeng.8b00848
Mittal M, Gupta A, Pandey OP (2018) Role of oxygen vacancies in Ag/Au doped CeO2 nanoparticles for fast photocatalysis. Sol Energy 165:206–216. https://doi.org/10.1016/j.solener.2018.03.033
Tian J, Sang Y, Zhao Z, et al. (2013) Enhanced photocatalytic performances of CeO2/TiO2 nanobelt heterostructures. 1–9. https://doi.org/10.1002/smll.201202346
Peternele WS, Monge Fuentes V, Fascineli ML et al. (2014) Experimental investigation of the coprecipitation method: an approach to obtain magnetite and maghemite nanoparticles with improved properties. J Nanomater 2014:1–11. https://doi.org/10.1155/2014/682985
Truffault L, Ta MT, Devers T et al. (2010) Application of nanostructured Ca doped CeO2 for ultraviolet filtration. Mater Res Bull 45:527–535. https://doi.org/10.1016/j.materresbull.2010.02.008
Truffault L, Yao QW, Wexler D et al. (2011) Synthesis and characterization of Fe doped CeO2 nanoparticles for pigmented ultraviolet filter applications. J Nanosci Nanotechnol 11:4019–4028. https://doi.org/10.1166/jnn.2011.3851
Priya R, Pandey OP (2019) Photoluminescent enhancement with co-doped alkali metals in Gd2O3:Eu synthesized by co-precipitation method and Judd Ofelt analysis. J Lumin 212:342–353. https://doi.org/10.1016/j.jlumin.2019.04.043
Nikam AV, Prasad BLV, Kulkarni AA (2018) Wet chemical synthesis of metal oxide nanoparticles: a review. CrystEngComm 20:5091–5107. https://doi.org/10.1039/C8CE00487K
Tan C, Zhang H (2015) Wet-chemical synthesis and applications of non-layer structured two-dimensional nanomaterials. Nat Commun 6:1–13. https://doi.org/10.1038/ncomms8873
Zarinkamar M, Farahmandjou M, Firoozabadi TP (2016) One-step synthesis of ceria (CeO2) nano-spheres by a simple wet chemical method. J Ceram Process Res 17:166–169. https://doi.org/10.36410/jcpr.2016.17.3.166
Ramanathan G, Rathan SV, Murali KR (2019) Photocatalytic activity of biosynthesized CeO2 nano particles. SN Appl Sci 1:1–10. https://doi.org/10.1007/s42452-018-0103-y
Sharma M, Kumar S, Pandey OP (2010) Study of energy transfer from capping agents to intrinsic vacancies/defects in passivated ZnS nanoparticles. J Nanopart Res 12:2655–2666. https://doi.org/10.1007/s11051-009-9844-2
Campisi S, Schiavoni M, Chan-Thaw CE, Villa A (2016) Untangling the role of the capping agent in nanocatalysis: recent advances and perspectives. Catalysts 6:1–21. https://doi.org/10.3390/catal6120185
Kusuma M, Chandrappa GT (2019) Effect of calcination temperature on characteristic properties of CaMoO 4 nanoparticles. J Sci Adv Mater Devices 4:150–157. https://doi.org/10.1016/j.jsamd.2019.02.003
Pal Singh RP, Hudiara IS, Rana SB (2016) Effect of calcination temperature on the structural, optical and magnetic properties of pure and Fe-doped ZnO nanoparticles. Mater Sci Pol 34:451–459. https://doi.org/10.1515/msp-2016-0059
Rondiya S, Rokade A, Jadhavar A et al. (2017) Effect of calcination temperature on the properties of CZTS absorber layer prepared by RF sputtering for solar cell applications. Mater Renew Sustain Energy 6:1–10. https://doi.org/10.1007/s40243-017-0092-6
Zia M. (2016) Effect of capping agents: structural, optical and biological properties of ZnO nanoparticles. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2016.06.042
Rudraswamy B. (2019) Effect of Ce3+ ion on structural and hyperfine interaction studies of Co0.5Ni0.5Fe2−xCexO4 ferrites: useful for permanent magnet applications. J Supercond Nov Magn 32:693–704.
Zhou XD, Huebner W (2001) Size-induced lattice relaxation in CeO2 nanoparticles. Appl Phys Lett 79:3512–3514. https://doi.org/10.1063/1.1419235
Wang B, Zhu B, Yun S, et al. (2019) Fast ionic conduction in semiconductor CeO2-δ electrolyte fuel cells. NPG Asia Mater 11. https://doi.org/10.1038/s41427-019-0152-8
Zhao R, Tian ZC, Zhao Z (2020) Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion. Green Process Synth 9:734–743. https://doi.org/10.1515/gps-2020-0053
Gharibshahi L, Saion E, Gharibshahi E et al. (2017) Structural and optical properties of Ag nanoparticles synthesized by thermal treatment method. Mater (Basel) 10:402. https://doi.org/10.3390/ma10040402
Lopez HF, Mendoza H (2013) Temperature effects on the crystallization and coarsening of nano-CeO2 powders. ISRN Nanomater 2013:1–7. https://doi.org/10.1155/2013/208614
Das A, Patra M, P MK, et al. (2020) Defect-induced visible-light-driven photocatalytic and photoelectrochemical performance of ZnO–CeO2 nanoheterojunctions. J Alloys Compd 157730. https://doi.org/10.1016/j.jallcom.2020.157730
Babitha KK, Sreedevi A, Priyanka KP et al. (2015) Structural characterization and optical studies of CeO2 nanoparticles synthesized by chemical precipitation. Indian J Pure Appl Phys 53:596–603
Khadar YAS, Balamurugan A, Devarajan VP, Subramanian R (2017) Hydrothermal synthesis of gadolinium (Gd) doped cerium oxide (CeO2) nanoparticles: characterization and antibacterial activity. Orient J Chem 33:2405–2411. https://doi.org/10.13005/ojc/330533
Vangelista S, Piagge R, Ek S, et al. (2017) Structural, chemical and optical properties of cerium dioxide fi lm prepared by atomic layer deposition on TiN and Si substrates. Thin Solid Films 636:78–84. https://doi.org/10.1016/j.tsf.2017.05.034
Jaidka S, Khan S, Singh K (2018) Na2O doped CeO2 and their structural, optical, conducting and dielectric properties. Phys B Condens Matter 550:189–198. https://doi.org/10.1016/j.physb.2018.08.036
Jun DU, Qi WU, Shan Z, et al. (2015) Effect of hydroxyl groups on hydrophilic and photocatalytic activities of rare earth doped titanium dioxide thin films. J Rare Earths 33:148–153. https://doi.org/10.1016/S1002-0721(14)60395-1
Munawaroh H, Sari PL, Wahyuningsih S, Ramelan AH. (2018) The photocatalytic degradation of methylene blue using graphene oxide (GO)/ZnO nanodrums. AIP Conference Proceedings 2014:020119.
Wu C-Y, Tu K-J, Deng J-P, Lo Y-S, Wu CH. (2017) Markedly enhanced surface hydroxyl groups of TiO2 nanoparticles with superior water-dispersibility for photocatalysis. Materials (Basel) 10:566. https://doi.org/10.3390/ma10050566
Pan Y, Shen X, Yao L, et al. (2018) Active sites in heterogeneous catalytic reaction on metal and metal oxide: theory and practice. Catalysts 8:478. https://doi.org/10.3390/catal8100478
Ganorkar RP, Kalkar KP, Tamgadge YS (2015) Synthesis, structural and optical properties of L-valine modified ZnO nanoparticles. Int J Chem Sci 13:2039–2048
Choudhury B, Chetri P, Choudhury A (2015) Annealing temperature and oxygen-vacancy- dependent variation of lattice strain, band gap and luminescence properties of CeO2 nanoparticles. J Exp Nanosci 10:103–114. https://doi.org/10.1080/17458080.2013.801566
Phoka S, Laokul P, Swatsitang E et al. (2009) Synthesis, structural and optical properties of CeO2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route. Mater Chem Phys 115:423–428. https://doi.org/10.1016/j.matchemphys.2008.12.031
Tamrakar R, Ramrakhiani M, Chandra BP (2008) Effect of capping agent concentration on photophysical properties of zinc sulfide nanocrystals. Open Nanosci J 2:12–16
Ungula J, Dejene BF (2016) Effect of solvent medium on the structural, morphological and optical properties of ZnO nanoparticles synthesized by the sol-gel method. Phys B Condens Matter 480:26–30. https://doi.org/10.1016/j.physb.2015.10.007
Mohanty B, Nayak J. (2017) Parameters dependent studies of structural, optical and electrical properties of CeO 2 nanoparticles prepared via facile one-pot hydrothermal technique. Mater Res Express 4. https://doi.org/10.1088/2053-1591/aa95e6
Singh Vig A, Gupta A, Pandey OP (2018) Efficient photodegradation of methylene blue (MB) under solar radiation by ZrC nanoparticles. Adv Powder Technol 29:2231–2242. https://doi.org/10.1016/j.apt.2018.06.007
Nurhasanah I, Sutanto H, Futikhaningtyas R (2014) Optical properties of Zn-doped CeO2 nanoparticles as function of Zn content. Adv Mater Res 896:108–111. https://doi.org/10.4028/www.scientific.net/AMR.896.108
Wang J, Wang Z, Huang B et al. (2012) Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl Mater Interfaces 4:4024–4030. https://doi.org/10.1021/am300835p
Mansingh S, Padhi DK, Parida KM (2016) Enhanced photocatalytic activity of nanostructured Fe doped CeO2 for hydrogen production under visible light irradiation. Int J Hydrog Energy 41:14133–14146. https://doi.org/10.1016/j.ijhydene.2016.05.191
Tiwari S, Balasubramanian N, Biring S, Sen S. (2018) Investigation of structural, optical and electronic properties of (Co, Ni) codoped CeO 2 nanoparticles. IOP Conf Ser Mater Sci Eng 390. https://doi.org/10.1088/1757-899X/390/1/012001
Hu C, Zhang Z, Liu H et al. (2006) Direct synthesis and structure characterization of ultrafine CeO 2 nanoparticles. Nanotechnology 17:5983–5987. https://doi.org/10.1088/0957-4484/17/24/013
Ramasamy V, Vijayalakshmi G (2015) Effect of Zn doping on structural, optical and thermal properties Of CeO2 nanoparticles. Superlattices Microstruct 85:510–521. https://doi.org/10.1016/j.spmi.2015.05.015
Khokhra R, Singh RK, Kumar R (2014) Effect of synthesis medium on aggregation tendencies of ZnO nanosheets and their superior photocatalytic performance. J Mater Sci 50:819–832. https://doi.org/10.1007/s10853-014-8642-0
Nanorods C, Liyanage AD, Perera SD et al. (2014) Synthesis, characterization, and photocatalytic activity of Y‑doped CeO2 nanorods. Am Chem Soc 4:577–584
Mangalam D, Manoharadoss D, Sadaiyandi K et al. (2016) Structural, optical, morphological and dielectric properties of cerium oxide nanoparticles 2. Experimental procedure 3. Results Discussion 19:478–482
Chahal S, Rani N, Kumar A, Kumar P (2019) UV-irradiated photocatalytic performance of yttrium doped ceria for hazardous Rose Bengal dye. Appl Surf Sci 493:87–93. https://doi.org/10.1016/j.apsusc.2019.06.284
Choudhury B, Choudhury A (2012) Ce 3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater Chem Phys 131:666–671. https://doi.org/10.1016/j.matchemphys.2011.10.032
Mekasuwandumrong O, Chaitaworn S, Panpranot J, Praserthdam P. (2019) Photocatalytic liquid-phase selective hydrogenation of 3-nitrostyrene to 3-vinylaniline of various treated-TiO2 without use of reducing gas. Catalysts 9. https://doi.org/10.3390/catal9040329
Duan H, Dong YZ, Huang Y et al. (2016) The important role of oxygen vacancies in Sr2MgSi2O7 phosphor. Phys Lett A 1:1–7. https://doi.org/10.1016/j.physleta.2016.01.001
Schilling C, Hofmann A, Hess C, Ganduglia-pirovano MV (2017) Raman spectra of polycrystalline CeO2: a density functional theory study. J Phys Chem C 121:20834–20849. https://doi.org/10.1021/acs.jpcc.7b06643
Reichling M, Torbru S. (2014) Evidence of subsurface oxygen vacancy ordering on reduced CeO2 (111) evidence of subsurface oxygen vacancy ordering on reduced CeO2 111. Phys Rev Lett 2. https://doi.org/10.1103/PhysRevLett.99.056101
Negi K, Umar A, Chauhan MS, Akhtar MS. (2019) Ag/CeO2 nanostructured materials for enhanced photocatalytic and antibacterial applications. Ceram Int 1. https://doi.org/10.1016/j.ceramint.2019.07.030
Hai WC, Hu TX, Rong LJ, Ying TS (2007) Preparation, characterization and photocatalytic activities of boron- and cerium-codoped TiO2. J Environ Sci 19:90–96. https://doi.org/10.1016/S1001-0742(07)60015-1
de Moraes NP, Valim RB, da Silva Rocha R et al. (2020) Effect of synthesis medium on structural and photocatalytic properties of ZnO/carbon xerogel composites for solar and visible light degradation of 4-chlorophenol and bisphenol A. Colloids Surf A Physicochem Eng Asp 584:124034. https://doi.org/10.1016/j.colsurfa.2019.124034
Sifontes AB, Rosales M, M‚ndez FJ (2013) Effect of calcination temperature on structural properties and photocatalytic activity of ceria nanoparticles synthesized employing chitosan as template. J Nanomater 2013:1–9
Niu Z, Li Y (2014) Removal and utilization of capping agents in nanocatalysis. Chem Mater 26:72–83. https://doi.org/10.1021/cm4022479
Jain N, Bhargava A, Panwar J (2014) Enhanced photocatalytic degradation of methylene blue using biologically synthesized “protein-capped” ZnO nanoparticles. Chem Eng J 243:549–555. https://doi.org/10.1016/j.cej.2013.11.085
Rakshit R, Khatun E, Pal M et al. (2017) Influence of functional group of dye on the adsorption behaviour of CoFe2O4 nano-hollow spheres. N J Chem 41:9095–9102. https://doi.org/10.1039/c7nj00941k
Gupta A, Mittal M, Singh MK et al. (2018) Low temperature synthesis of NbC/C nano-composites as visible light photoactive catalyst. Sci Rep 8:1–17. https://doi.org/10.1038/s41598-018-31989-z
Rajrana K, Gupta A, Mir RA, Pandey OP (2019) Facile sono-chemical synthesis of nanocrystalline MnO2 for catalytic and capacitive applications. Phys B Condens Matter 564:179–185. https://doi.org/10.1016/j.physb.2019.04.002
Bayu A, Nandiyanto D, Zaen R, Oktiani R. (2017) Correlation between crystallite size and photocatalytic performance of micrometer-sized Monoclinic WO3 particles. Arab J Chem https://doi.org/10.1016/j.arabjc.2017.10.010
Saha D, Desipio MM, Hoinkis TJ et al. (2018) Influence of hydrogen peroxide in enhancing photocatalytic activity of carbon nitride under visible light: an insight into reaction intermediates. J Environ Chem Eng 6:1–28
Pouretedal HR, Kadkhodaie A (2010) Synthetic CeO2 nanoparticle catalysis of methylene blue photodegradation: kinetics and mechanism. Chin J Catal 31:1328–1334. https://doi.org/10.1016/S1872-2067(10)60121-0
Huda A, Ichwani R, Handoko CT, et al. (2019) Comparative photocatalytic performances towards acid yellow 17 (AY17) and direct blue 71 (DB71) degradation using Sn3O4 flower-like structure. J Phys Conf Ser 1282. https://doi.org/10.1088/1742-6596/1282/1/012097
Chen X, Wu Z, Liu D, Gao Z (2017) Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res Lett 12:4–13. https://doi.org/10.1186/s11671-017-1904-4
Acknowledgements
The authors are thankful to AIRF JNU, New Delhi for TEM and Raman results and SAIF Panjab University, Chandigarh for XRD and FTIR results. The authors are thankful to Dr Aayush Gupta and Ruby Priya for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sharma, P.K., Pandey, O.P. Effect of processing parameters on structural and optical properties of CeO2 nanoparticles for the removal of crystal violet dye. J Sol-Gel Sci Technol 99, 75–91 (2021). https://doi.org/10.1007/s10971-021-05542-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05542-w