Abstract
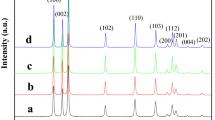
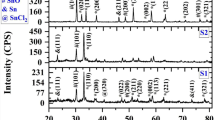
A simple soft chemical method has been suggested for large-scale production of zinc oxide (ZnO) nanosheets at room temperature using two synthesis mediums: aqueous (H2O) and non-aqueous (C2H5OH). In H2O medium, nanosheets interwoven group wise in flower-like structures revealing the strong inter-hydrogen bonding among initially nucleated ZnO nanocrystals, whereas weak hydrogen bonding in C2H5OH medium leads to the formation of un-aggregated interwoven’ nanosheets. The growth of ZnO flower-like and interwoven nanosheets proceeded via anisotropic oriented attachment of ZnO nanocrystals. Obtained nanosheets were faceted, possessing large surface area, width hundreds of nanometers, and thickness in tens of nanometer, as characterized by scanning electron microscopy and transmission electron microscopy. These nanosheets show high sunlight photocatalytic activity toward the degradation of an organic pollutant ‘methylene blue dye.’ The enhancement in photodegradation efficiencies, interwoven sheets 99.94 %, and flower-like nanosheets 79.76 % for 120 min of irradiation is attributed to the surface oxygen vacancies narrowing the band gap as confirmed by photoluminescence spectra, faceted geometry, and large surface area of ZnO nanosheets.











Similar content being viewed by others
References
Gonzalez-Valls I, Lira-Cantu M (2009) Vertically-aligned nanostructures of ZnO for excitonic solar cells: a review. Energy Environ Sci 2:19–34
Lai E, Kim W, Yang P (2008) Vertical nanowire array-based light emitting diode. Nano Res 1:123–128
Jin Y, Wang J, Sun B, Blakesley JC, Greenham NC (2008) Solution-processed ultraviolet photodetectors based on colloidal ZnO nanoparticles. Nano Lett 8(6):1649–1653
Zhang C, Zhang F, Xia T, Kumar N, Hahm JI, Liu J, Wang ZL, Xu J (2009) Low-threshold two-photon pumped ZnO nanowire lasers. Opt Express 17(10):7893–7900
Wei A, Wang Z, Pan LH, Li WW, Xiong L, Dong XC, Huang W (2011) Room-temperature NH3 gas sensor based on hydrothermally grown ZnO nanorods. Chin Phys Lett 28:080702
Frenzel H, Lajn A, Wenckstern H, Lorenz M, Schein F, Zhang Z, Grundmann M (2010) Recent progress on Zno-based metal-semiconductor field-effect transistors and their application in transparent integrated circuits. Adv Mater 22:5332–5349
Hillman TR, Yamauchi T, Choi W, Dasari RR, Feld MS, Park Y, Yaqoob Z (2013) Digital optical phase conjugation for delivering two-dimensional images through turbid media. Sci Rep 3:1909
Choi Y, Yang TD, Fang-Yen C, Kang P, Lee KJ, Dasari RR, Feld MS, Choi W (2011) Overcoming the diffraction limit using multiple light scattering in a highly disordered medium. Phys Rev Lett 104:023902
Khokhra R, Kumar M, Rawat N, Barman PB, Jang H, Kumar R, Lee H (2013) Enhancing the numerical aperture of lenses using ZnO nanostructure-based turbid media. J Opt 15:125714
Garcia MA, Merino JM, Fernandez EP, Quesada A, Venta J (2007) Magnetic properties of ZnO nanoparticles. Nano Lett 7(6):1489–1494
Lim JH, Kang CK, Kim KK, Park IK, Hwang DK, Park SJ (2006) Electroluminescence emission from ZnO light-emitting diodes grown by high-temperature radiofrequency sputtering. Adv Mater 18:2720–2724
Lansdown ABG, Taylor A (1997) Zinc and titanium oxides: promising UV-absorbers but what influence do they have on the intact skin? Int J Cosmet Sci 19:167–172
Mohammad MT, Hashim AA, Al-Maamory MH (2006) Highly conductive and transparent ZnO thin films prepared by spray pyrolysis technique. Mater Chem Phys 99:382–387
Vempati S, Mitra J, Dawson P (2012) One-step synthesis of ZnO nanosheets: a blue-white fluorophore. Nanoscale Res Lett 7:470
Wang J, Liu P, Fu X, Li Z, Han W, Wang X (2009) Relationship between oxygen defects and the photocatalytic property of ZnO nanocrystals in Nafion membranes. Langmuir 25(2):1218–1223
Guo MY, Ng AMC, Liu FZ, Djurisic AB, Chan WK, Su HM, Wong KS (2011) Effect of native defects on photocatalytic properties of ZnO. J Phys Chem C 115:11095–11101
Li GR, Hu T, Pan GL, Yan TY, Gao XP, Zhu HY (2008) Morphology-function relationship of ZnO: polar planes, oxygen vacancies, and activity. J Phys Chem C 112:11859–11864
Becker J, Raghupathi KR, St. Pierre J, Zhao D, Koodali RT (2011) Tuning of the crystallite and particle sizes of ZnO nanocrystalline materials in solvothermal synthesis and their photocatalytic activity for dye degradation. J Phys Chem C 115:13844–13850
McLaren A, Valdes-Solis T, Li G, Tsang SC (2009) Shape and size effects of ZnO nanocrystals on photocatalytic activity. J Am Chem Soc 131(35):12540–12541
Jang ES, Won JH, Hwang SJ, Choy JH (2006) Fine tuning of the face orientation of ZnO crystals to optimize their photocatalytic activity. Adv Mater 18:3309–3312
Tian ZR, Voigt JA, Liu J, Mckenzie B, Mcdermott MJ, Rodriguez MA, Konishi H, Xu H (2003) Complex and oriented ZnO nanostructures. Nat Mater 2(12):821–826
Wang L, Chang LX, Zhao B, Yuan ZY, Shao GS, Zheng WJ (2008) Systematic investigation on morphologies, forming mechanism, photocatalytic and photoluminescent properties of ZnO nanostructures constructed in ionic liquids. Inorg Chem 47:1443–1452
Zhang L, Yang HQ, Ma JH, Li L, Wang XW, Zhang LH, Tian S, Wang XY (2010) Controllable synthesis and shape-dependent photocatalytic activity of ZnO nanorods with a cone and different aspect ratios and of short and fat ZnO microrods by varying the reaction temperature and time. Appl Phys A 100(4):1061–1067
Kong M, Li Y, Chen X, Tian T, Fang P, Zheng F, Zhao X (2011) Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J Am Chem Soc 133(41):16414–16417
Justicia I, Ordejon P, Canto G, Mozos JL, Fraxedes J, Battiston GA, Gerbasi R, Figueras A (2002) Designed self-doped titanium oxide thin films for efficient visible-light photocatalysis. Adv Mater 14(19):1399–1402
Zuo F, Wang L, Wu T, Zhang ZY, Borchardt D, Feng PY (2010) Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J Am Chem Soc 132(34):11856–11857
Wan Q, Wang TH, Zhao JC (2005) Enhanced photocatalytic activity of ZnO nanotetrapods. Appl Phys Lett 87(8):083105
Koch U, Fojtik A, Weller H, Henglein A (1985) Photochemistry of semiconductor colloids. Preparation of extremely small ZnO particles, fluorescence phenomena and size quantization effects. Chem Phys Lett 122(5):507–510
Hynek J, Kalousek V, Žouželka R, Bezdička P, Dzik P, Rathouský J, Demel J, Lang K (2014) High photocatalytic activity of transparent films composed of ZnO nanosheets. Langmuir 30(1):380–386
Bang S, Lee S, Ko Y, Park J, Shin S, Seo H, Jeon H (2012) Photocurrent detection of chemically tuned hierarchical ZnO nanostructures grown on seed layers formed by atomic layer deposition. Nanoscale Res Lett 7(1):290
Guo L, Ji YL, Xu H, Simon P, Wu Z (2002) Regularly shaped, single-crystalline ZnO nanorods with Wurtzite structure. J Am Chem Soc 124(50):14864–14865
Munoz-Espi R, Jeschke G, Lieberwirth I, Gomez CM, Wegner G (2007) ZnO-latex hybrids obtained by polymer-controlled crystallization: a spectroscopic investigation. J Phys Chem B 111(4):697–707
Pacholski C, Kornowski A, Weller H (2002) Self-assembly of ZnO: from nanodots to nanorods. Angew Chem Int Ed 41(7):1188–1191
Taubert A, Glasser G, Palms D (2002) Kinetics and particle formation mechanism of zinc oxide particles in polymer-controlled precipitation from aqueous solution. Langmuir 18(11):4488–4494
Tian ZR, Liu J, Voigt JA, Mckenzie B, Xu H (2003) Hierarchical and self-similar growth of self-assembled crystals. Angew Chem Int Ed 42(4):413–417
Umetsu M, Mizuta M, Tsumoto K, Ohara S, Takami S, Watanabe H, Kumagai I, Adschiri T (2005) Bioassisted room-temperature immobilization and mineralization of zinc oxide-the structural ordering of ZnO nanoparticles into a flower-type morphology. Adv Mater 17(21):2571–2575
Zhang T, Dong W, Keeter-Brewer M, Konar S, Njabon RN, Tian ZR (2006) Site-specific nucleation and growth kinetics in hierarchical nanosyntheses of branched ZnO crystallites. J Am Chem Soc 128(33):10960–10968
Zhang Y, Mu J (2007) Controllable synthesis of flower- and rod-like ZnO nanostructures by simply tuning the ratio of sodium hydroxide to zinc acetate. Nanotechnology 18(7):075606
Zou H, Luan Y, Ge J, Wang Y, Zhuang G, Li R, Li Z (2011) Synthesis of ZnO particles on zinc foil in ionic-liquid precursors. CrystEngComm 13(7):2656–2660
Zhang J, Wang J, Zhou S, Duan K, Feng B, Weng J, Tang H, Wu P (2010) Ionic liquid-controlled synthesis of ZnO microspheres. J Mater Chem 20:9798–9804
Kahn ML, Monge M, Colliere V, Senocq F, Maisonnat A, Chaudret B (2005) Size and shape control of crystalline zinc oxide nanoparticles: a new organometallic synthetic method. Adv Funct Mater 15(3):458–468
Monge M, Kahn ML, Maisonnat A, Chaudret B (2003) Room-temperature organometallic synthesis of soluble and crystalline ZnO nanoparticles of controlled size and shape. Angew Chem Int Ed 42(43):5321–5324
Antonietti M, Kuang D, Smarsly B, Zhou Y (2004) Ionic liquids for the convenient synthesis of functional nanoparticles and other inorganic nanostructures. Angew Chem Int Ed 43(38):4988–4992
Zhang J, Sun L, Yin J, Su H, Liao C, Yan C (2002) Control of ZnO morphology via a simple solution route. Chem Mater 14(10):4172–4177
Zhang X, Qin J, Xue Y, Yu P, Zhang B, Wang L, Liu R (2014) Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci Rep 4:4596
Yang H, Cai W, Guo X (2014) Preparation and infrared emissivities of self-assembled ZnO spherical aggregates. Mater Sci Semicond Process 24:164–168
Liu D, Lv Y, Zhang M, Liu Y, Zhu Y, Zong R, Zhu Y (2014) Defect-related photoluminescence and photocatalytic properties of porous ZnO nanosheets. J Mater Chem A 2:15377–15388
Bai W, Zhu X, Zhu Z, Chu J (2008) Synthesis of zinc oxide nanosheet thin films and their improved field emission and photoluminescence properties by annealing processing. Appl Surf Sci 254(20):6483–6488
Cao B, Cai W, Li Y, Sun F, Zhang L (2005) Ultraviolet-light-emitting ZnO nanosheets prepared by a chemical bath deposition method. Nanotechnology 16(9):1734
Sun Y, Wang L, Yu X, Chen K (2012) Facile synthesis of flower-like 3D ZnO superstructures via solution route. CrystEngComm 14:3199–3204
Wang J, Hou S, Zhang L, Chen J, Xiang L (2014) Ultra-rapid formation of ZnO hierarchical structures from dilution-induced supersaturated solutions. CrystEngComm 16:7115–7123
Ma J, Su S, Fu W, Yang H, Zhou X, Huizhen Yao H, Chen Y, Yang L, Sun M, Mu Y, Lv P (2014) Synthesis of ZnO nanosheet array film with dominant 0001 facets and enhanced photoelectrochemical performance co-sensitized by CdS/CdSe. CrystEngComm 16:2910–2916
Xingfu Z, Zhaolin H, Yiqun F, Su C, Weiping D, Nanping X (2008) Microspheric organization of multilayered ZnO nanosheets with hierarchically porous structures. J Phys Chem C 112(31):11722–11728
Khoa NT, Kim SW, Thuan DV, Yoo DH, Kim EJ, Hahn SH (2014) Hydrothermally controlled ZnO nanosheet self-assembled hollow spheres/hierarchical aggregates and their photocatalytic activities. CrystEngComm 16:1344–1350
Banfield JF, Welch SA, Zhang H, Ebert TT, Penn RL (2000) Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 289(5480):751–754
Li P, Liu H, Zhang YF, Wei Y, Wang XK (2007) Synthesis of flower-like ZnO microstructures via a simple solution route. Mater Chem Phys 106(1):63–69
Jia B, Gao L (2008) Growth of well-defined cubic hematite single crystals: oriented aggregation and ostwald ripening. Cryst Growth Des 8(4):1372–1376
Vinogradov SN, Linnell RH (1971) Hydrogen bonding. Van Nostrand Reinhold Co, New York
Viswanatha R, Sarma DD (2007) Growth of nanocrystals in solution. In: Rao CNR, Müller A, Cheetham AK (eds) Nanomaterials chemistry: recent developments and new directions. Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim, pp 140–157
Kawska A, Duchstein P, Hochrein O, Zahn D (2008) Atomistic mechanisms of ZnO aggregation from ethanolic solution: ion association, proton transfer, and self-organization. Nano Lett 8(8):2336–2340
Mo M, Yu JC, Zhang LZ, Li SKA (2005) Self-assembly of ZnO nanorods and nanosheets into hollow microhemispheres and microspheres. Adv Mater 17(6):756–760
Liu Y, Wang D, Peng Q, Chu D, Liu X, Li Y (2011) Directly assembling ligand-free ZnO nanocrystals into three-dimensional mesoporous structures by oriented attachment. Inorg Chem 50(12):5841–5847
Layek A, Mishra G, Sharma A, Spasova M, Dhar S, Chowdhury A, Bandyopadhyaya R (2012) A Generalized three-stage mechanism of ZnO nanoparticle formation in homogeneous liquid medium. J Phys Chem C 116(46):24757–24769
Li WJ, Shi EW, Zhong WZ, Yin ZW (1999) Growth mechanism and growth habit of oxide crystals. J Cryst Growth 203(1–2):186–196
Zhang DF, Sun LD, Yin JL, Yan CH, Wang RM (2005) Attachment-driven morphology evolvement of rectangular ZnO nanowires. J Phys Chem B 109(18):8786–8790
Xu S, Wang ZL (2011) One-dimensional ZnO nanostructures: solution growth and functional properties. Nano Res 4(11):1013–1098
Cheng B, Shi W, Russell-Tanner JM, Zhang L, Samulski ET (2006) Synthesis of variable aspect-ratio, single-crystalline ZnO nanostructures. Inorg Chem 45(3):1208–1214
Xu F, Shen Y, Sun L, Zeng H, Lu Y (2011) Enhanced photocatalytic activity of hierarchical ZnO nanoplate-nanowire architecture as environmentally safe and facilely recyclable photocatalyst. Nanoscale 3:5020–5025
Liao ZM, Lu Y, Wu HC, Bie YQ, Zhou YB, Yu DP (2011) Improved performance of ZnO nanowire field-effect transistors via focused ion beam treatment. Nanotechnology 22(37):375201
Zhang AQ, Zhang L, Sui L, Qian DJ, Chen M (2013) Morphology-controllable synthesis of ZnO nano/micro structures by a solvothermal process in ethanol solution. Cryst Res Technol 48(11):947–955
García-Rodríguez S (2013) Alternative metal oxide photocatalysts. In: Coronado JM, Fresno F, Hernández-Alonso MD, Portela R (eds) Design of advanced photocatalytic materials for energy and environmental applications. Springer, London, p 106
Miao TT, Guo YR, Pan QJ (2013) The SL-assisted synthesis of hierarchical ZnO nanostructures and their enhanced photocatalytic activity. J Nanopart Res 15(6):1725
Eskizeybek V, Sarı F, Gulce H, Gulce A, Avc A (2012) Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Appl Catal B 119–120:197–206
Seema H, Kemp KC, Chandra V, Kim KS (2012) Graphene–SnO2 composites for highly efficient photocatalytic degradation of methylene blue under sunlight. Nanotechnology 23(35):355705
Pons AJ, Sagués F, Bees MA, Sørensen PG (2000) Pattern formation in the methylene-blue–glucose system. J Phys Chem B 104(10):2251–2259
Dave MD, Pande UC (2013) Photoinduced electron transfer reaction of 2-thiobarbituric acid and methylene blue: mechanism and kinetics. Chem Sci Trans 2(3):749–760
Matsuoka M (1990) Other chromophores. In: Matsuoka M (ed) Infrared absorbing dyes. Plenum Press, New York, p 89
Salvador P, Garcia Gonzalez ML, Munoz F (1992) Catalytic role of lattice defects in the photoassisted oxidation of water at (001) n-titanium(IV) oxide rutile. J Phys Chem 96(25):10349–10353
Liu Y, Son WJ, Lu J, Huang B, Dai Y, Whangbo MH (2011) Composition dependence of the photocatalytic activities of BiOCl1−x Br x solid solutions under visible light. Chem Eur J 17(34):9342–9349
Warule SS, Chaudhari NS, Kale BB, More MA (2009) Novel sonochemical assisted hydrothermal approach towards the controllable synthesis of ZnO nanorods, nanocups and nanoneedles and their photocatalytic study. CrystEngComm 11:2776–2783
Yu W, Li X, Gao X (2005) Catalytic synthesis and structural characteristics of high-quality tetrapod-like ZnO nanocrystals by a modified vapor transport process. Cryst Growth Des 5(1):151–155
Liu B, Fu Z, Jia Y (2001) Green luminescent center in undoped zinc oxide films deposited on silicon substrates. Appl Phys Lett 79(7):943
Mahamuni S, Borgohain K, Bendre BS, Leppert VJ, Risbud SH (1999) Spectroscopic and structural characterization of electrochemically grown ZnO quantum dots. J Appl Phys 85(5):2861
Hu JQ, Ma XL, Xie ZY, Wong NB, Lee CS, Lee ST (2001) Characterization of zinc oxide crystal whiskers grown by thermal evaporation. Chem Phys Lett 344(1–2):97–100
Williams G, Kamat PV (2009) Graphene semiconductor nanocomposites: excited-state interactions between ZnO nanoparticles and graphene oxide. Langmuir 25(24):13869–13873
Lu Y, Wang L, Wang D, Xie T, Chen L, Lin Y (2011) A comparative study on plate-like and flower-like ZnO nanocrystals surface photovoltage property and photocatalytic activity. Mater Chem Phys 129(1–2):281–287
Umar A, Chauhan MS, Chauhan S, Kumar R, Kumar G, Al-Sayari SA, Hwang SW, Al-Hajry A (2011) Large-scale synthesis of ZnO balls made of fluffy thin nanosheets by simple solution process: structural, optical and photocatalytic properties. J Colloid Interface Sci 363(2):521–528
Tong Y, Cheng J, Liu Y, Siu GG (2009) Enhanced photocatalytic performance of ZnO hierarchical nanostructures synthesized via a two-temperature aqueous solution route. Scr Mater 60(12):1093–1096
Sun L, Shao R, Chen Z, Tang L, Dai Y, Ding J (2012) Alkali-dependent synthesis of flower-like ZnO structures with enhanced photocatalytic activity via a facile hydrothermal method. Appl Surf Sci 258(14):5455–5461
Acknowledgements
This work was supported by the research fund of Nanotechnology Lab, Jaypee University of Information Technology, Waknaghat, Solan (H.P.), India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khokhra, R., Singh, R.K. & Kumar, R. Effect of synthesis medium on aggregation tendencies of ZnO nanosheets and their superior photocatalytic performance. J Mater Sci 50, 819–832 (2015). https://doi.org/10.1007/s10853-014-8642-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8642-0