Abstract
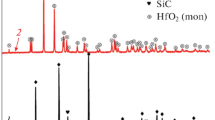
Using the sol–gel process, a composite powder of HfB2–(SiO2–C) composition was synthesized and then utilized for reactive sintering (hot pressing, 30 MPa; 1800 °C, 15 min) of ultra-high-temperature ceramic HfB2–30 vol% SiC composites. It was established that nanocrystalline silicon carbide (36 ± 2 nm) in a cubic modification is formed during hot pressing; HfO2 and HfC impurities were not found. The relative density of the obtained materials was 89.2 ± 2.3%. The long-term (40-min) oxidation resistance of the HfB2–30 vol% SiC sample was studied under the exposure to the supersonic dissociated airflow on a high-frequency induction plasmatron (heat flux changed from 232 to 779 W/cm2, pressure in the plasmatron chamber was 15 hPa), in a configuration that prevented significant heat discharge from the sample to a water-cooled holder (with 1-mm overhang). It was shown that, with a stepwise increase in the heat load in the initial stages, the temperature distribution over the front surface of the sample was relatively uniform. However, at the heat flux of 598 W/cm2, local overheated areas appeared in the central region, which spread over almost the entire surface of the sample within 1–2 min; the average temperature was ~2560 °C. Using emission spectroscopy data from the boundary layer above the sample surface, as well as XRD and SEM of the sample after exposure, it was shown that the sharp increase in temperatures from ~1500–1600 to 2500–2600 °C was associated with a change in the chemical nature of the surface, due to evaporation of borosilicate glass components and the appearance of a porous highly catalytic HfO2 with low thermal conductivity on the surface. It was noted that these processes under the exposure to the supersonic flow started at lower temperature than under the exposure to subsonic dissociated airflows.

Using the sol-gel process, a composite powder of HfB2-(SiO2-C) composition was synthesized and then utilized during the reactive sintering (hot pressing, 30 MPa; 1800 °C, 15 min) of ultra-high-temperature HfB2-30 vol% SiC ceramic composites. The long-term (40 min) oxidation resistance of the HfB2-30 vol% SiC sample was studied under the exposure to the supersonic dissociated air flow on a high-frequency induction plasmatron (heat flux changed from 232 to 779 W/cm2, pressure in the plasmatron chamber was 15 hPa), in a configuration that prevented significant heat discharge from the sample to a water cooled holder (with 1 mm overhang).
Highlights
-
HfB2–(SiO2–C) composite powder was synthesized using the sol–gel process.
-
HfB2–30 vol% SiC UHTC was produced by reactive sintering of HfB2–(SiO2–C) powder.
-
Oxidation resistance of UHTC under the exposure to the supersonic airflow at 2500 °C was studied.
-
Features of material oxidation were identified, in particular utilizing emission spectroscopy of the boundary layer.
-
The composition and microstructure of the oxidized material layer were studied, both its surface and thin section.













Similar content being viewed by others
References
Simonenko EP, Sevast’yanov DV, Simonenko NP et al. (2013) Promising ultra-high-temperature ceramic materials for aerospace applications. Russ J Inorg Chem 58:1669–1693. https://doi.org/10.1134/S0036023613140039
Zoli L, Vinci A, Galizia P et al. (2018) On the thermal shock resistance and mechanical properties of novel unidirectional UHTCMCs for extreme environments. Sci Rep 8:9148. https://doi.org/10.1038/s41598-018-27328-x
Silvestroni L, Failla S, Vinokurov V et al. (2019) Core-shell structure: An effective feature for strengthening ZrB2 ceramics. Scr Mater 160:1–4. https://doi.org/10.1016/j.scriptamat.2018.09.024
Cheng Y, Lyu Y, Han W et al. (2018) Multiscale toughening of ZrB2‐SiC‐Graphene@ZrB2‐SiC dual composite ceramics. J Am Ceram Soc jace.16068. https://doi.org/10.1111/jace.16068
Grashchenkov DV, Sorokin OY, Lebedeva YE, Vaganova ML (2015) Specific features of sintering of HfB2-based refractory ceramic by hybrid spark plasma sintering. Russ J Appl Chem 88:386–393. https://doi.org/10.1134/S1070427215030040
Simonenko EP, Simonenko NP, Gordeev AN et al. (2018) Study of the thermal behavior of wedge-shaped samples of HfB2–45 vol % SiC ultra-high-temperature composite in a high-enthalpy air flow. Russ J Inorg Chem 63:421–432. https://doi.org/10.1134/S0036023618040186
Cissel KS, Opila E (2018) Oxygen diffusion mechanisms during high-temperature oxidation of ZrB2-SiC. J Am Ceram Soc 101:1765–1779. https://doi.org/10.1111/jace.15298
Monteverde F, Cecere A, Savino R (2017) Thermo-chemical surface instabilities of SiC-ZrB2 ceramics in high enthalpy dissociated supersonic airflows. J Eur Ceram Soc 37:2325–2341. https://doi.org/10.1016/j.jeurceramsoc.2017.01.018
Feng X, Wang X, Liu Y et al. (2018) Pursuing enhanced oxidation resistance of ZrB2 ceramics by SiC and WC co-doping. J Eur Ceram Soc 38:5311–5318. https://doi.org/10.1016/j.jeurceramsoc.2018.07.041
Inoue R, Arai Y, Kubota Y et al. (2018) Oxidation of ZrB2 and its composites: a review. J Mater Sci 53:14885–14906. https://doi.org/10.1007/s10853-018-2601-0
Iatsyuk IV, Pogozhev YS, Levashov EA et al. (2018) Combustion synthesis of high-temperature ZrB2-SiC ceramics. J Eur Ceram Soc 38:2792–2801. https://doi.org/10.1016/j.jeurceramsoc.2018.02.016
Guo S (2018) High-temperature mechanical behavior of ZrB2-based composites with micrometer- and nano-sized SiC particles. J Am Ceram Soc 101:2707–2711. https://doi.org/10.1111/jace.15446
Silvestroni L, Failla S, Neshpor I, Grigoriev O (2018) Method to improve the oxidation resistance of ZrB2-based ceramics for reusable space systems. J Eur Ceram Soc 38:2467–2476. https://doi.org/10.1016/j.jeurceramsoc.2018.01.025
Sevastyanov VG, Simonenko EP, Gordeev AN et al. (2015) Behavior of a sample of the ceramic material HfB2–SiC (45 vol %) in the flow of dissociated air and the analysis of the emission spectrum of the boundary layer above its surface. Russ J Inorg Chem 60:1360–1373. https://doi.org/10.1134/S0036023615110133
Simonenko EP, Gordeev AN, Simonenko NP et al. (2016) Behavior of HfB2-SiC (10, 15, and 20 vol%) ceramic materials in high-enthalpy air flows. Russ J Inorg Chem 61:1203–1218. https://doi.org/10.1134/S003602361610017X
Mashhadi M, Khaksari H, Safi S (2015) Pressureless sintering behavior and mechanical properties of ZrB2–SiC composites: effect of SiC content and particle size. J Mater Res Technol 4:416–422. https://doi.org/10.1016/j.jmrt.2015.02.004
Kim S, Chae J-M, Lee S-M et al. (2013) Thermal and mechanical properties of ZrB2-SiC ceramics fabricated by hot pressing with change in ratio of submicron to nano size of SiC. J Korean Ceram Soc 50:410–415. https://doi.org/10.4191/kcers.2013.50.6.410
Azizian-Kalandaragh Y, Namini AS, Ahmadi Z, Shahedi Asl M (2018) Reinforcing effects of SiC whiskers and carbon nanoparticles in spark plasma sintered ZrB2 matrix composites. Ceram Int 44:19932–19938. https://doi.org/10.1016/j.ceramint.2018.07.258
Liu Q, Han W, Han J (2010) Influence of SiCnp content on the microstructure and mechanical properties of ZrB2–SiC nanocomposite. Scr Mater 63:581–584. https://doi.org/10.1016/j.scriptamat.2010.06.005
Parvizi S, Ahmadi Z, Zamharir MJ, Shahedi Asl M (2018) Synergistic effects of graphite nano-flakes and submicron SiC particles on the characteristics of spark plasma sintered ZrB2 nanocomposites. Int J Refract Met Hard Mater 75:10–17. https://doi.org/10.1016/j.ijrmhm.2018.03.017
Jaberi Zamharir M, Shahedi Asl M, Ghassemi Kakroudi M et al. (2015) Significance of hot pressing parameters and reinforcement size on sinterability and mechanical properties of ZrB2–25vol% SiC UHTCs. Ceram Int 41:9628–9636. https://doi.org/10.1016/j.ceramint.2015.04.027
Han W, Zhou S, Zhang J (2014) Single-cycle thermal shock resistance of ZrB2–SiCnp ceramic composites. Ceram Int 40:16665–16669. https://doi.org/10.1016/j.ceramint.2014.08.028
Kim S, Chae J-M, Lee S-M et al. (2014) Change in microstructures and physical properties of ZrB2–SiC ceramics hot-pressed with a variety of SiC sources. Ceram Int 40:3477–3483. https://doi.org/10.1016/j.ceramint.2013.09.082
Kováčová Z, Bača Ľ, Neubauer E, Kitzmantel M (2016) Influence of sintering temperature, SiC particle size and Y2O3 addition on the densification, microstructure and oxidation resistance of ZrB2–SiC ceramics. J Eur Ceram Soc 36:3041–3049. https://doi.org/10.1016/j.jeurceramsoc.2015.12.028
Simonenko EP, Simonenko NP, Ezhov YS et al. (2015) Study of the synthesis of nanocrystalline mixed tantalum–zirconium carbide. Phys At Nucl 78:1357–1365. https://doi.org/10.1134/S106377881512011X
Simonenko EP, Ignatov NA, Simonenko NP et al. (2011) Synthesis of highly dispersed super-refractory tantalum-zirconium carbide Ta4ZrC5 and tantalum-hafnium carbide Ta4HfC5 via sol-gel technology. Russ J Inorg Chem 56:1681–1687. https://doi.org/10.1134/S0036023611110258
Simonenko EP, Simonenko NP, Derbenev AV et al. (2013) Synthesis of nanocrystalline silicon carbide using the sol-gel technique. Russ J Inorg Chem 58:1143–1151. https://doi.org/10.1134/S0036023613100215
Sevast’yanov VG, Simonenko EP, Ignatov NA et al. (2010) Low-temperature synthesis of TaC through transparent tantalum-carbon containing gel. Inorg Mater 46:495–500. https://doi.org/10.1134/S0020168510050109
Sevastyanov VG, Simonenko EP, Ignatov NA et al. (2011) Low-temperature synthesis of nanodispersed titanium, zirconium, and hafnium carbides. Russ J Inorg Chem 56:661–672. https://doi.org/10.1134/S0036023611050214
Liu B, Ju W, Zhang J et al. (2017) Improvement of mechanical strength of ultralight resorcinol–formaldehyde/silica aerogel by addition of zirconia. J Sol-Gel Sci Technol 83:100–108. https://doi.org/10.1007/s10971-017-4400-5
Najafi A, Golestani-Fard F, Rezaie HR, Ehsani N (2011) A study on sol–gel synthesis and characterization of SiC nano powder. J Sol-Gel Sci Technol 59:205–214. https://doi.org/10.1007/s10971-011-2482-z
Najafi A, Golestani-Fard F, Rezaie HR (2015) Improvement of SiC nanopowder synthesis by sol–gel method via TEOS/resin phenolic precursors. J Sol-Gel Sci Technol 75:255–263. https://doi.org/10.1007/s10971-015-3695-3
Shawgi N, Xi LiS, Wang S et al. (2017) Synthesis of nano particles and fiber-like shape boron carbide powder from ploy (vinyl alcohol) and boric acid. J Sol-Gel Sci Technol 82:450–457. https://doi.org/10.1007/s10971-017-4320-4
Zhang X, Song B, Zhang Y et al. (2018) Influences of pre-forming on preparation of SiC by microwave heating. Ceram Int 44:21309–21313. https://doi.org/10.1016/j.ceramint.2018.08.182
Simonenko EP, Simonenko NP, Papynov EK et al. (2017) Preparation of porous SiC-ceramics by sol–gel and spark plasma sintering. J Sol-Gel Sci Technol 82:748–759. https://doi.org/10.1007/s10971-017-4367-2
Simonenko EP, Simonenko NP, Sevastyanov DV et al. (2016) Preparation of MB2/SiC and MB2/SiC-MC (M=Zr or Hf) powder composites which are promising materials for design of ultra-high-temperature ceramics. Russ J Inorg Chem 61:1649–1676. https://doi.org/10.1134/S0036023616130039
Simonenko EP, Simonenko NP, Sevastyanov VG, Kuznetsov NT (2016) Preparation of HfB2/SiC composite powders by sol–gel technology. Russ J Inorg Chem 61:1483–1498. https://doi.org/10.1134/S0036023616120172
Zhao B, Zhang Y, Li J et al. (2013) Morphology and mechanism study for the synthesis of ZrB2–SiC powders by different methods. J Solid State Chem 207:1–5. https://doi.org/10.1016/j.jssc.2013.08.028
Zhang Y, Zhang Y, Li R-X et al. (2015) Synthesis of ZrB2–SiC composite powders by sol–gel method using acetic acid as chemical modifier. J Taiwan Inst Chem Eng 46:200–204. https://doi.org/10.1016/j.jtice.2014.09.022
Patra N, Jayaseelan DD, Lee WE (2016) Synthesis of ZrB2/SiC composite powders by single-step solution process from organic–inorganic hybrid precursor. Adv Appl Ceram 115:36–42. https://doi.org/10.1179/1743676115Y.0000000058
Cao Y, Zhang H, Li F et al. (2015) Preparation and characterization of ultrafine ZrB2–SiC composite powders by a combined sol–gel and microwave boro/carbothermal reduction method. Ceram Int 41:7823–7829. https://doi.org/10.1016/j.ceramint.2015.02.117
Che XP, Zhu SZ, Yang LJ, Xu Q (2010) Solution-based synthesis of ultra-fine ZrB2 powders and ZrB2-SiC composite powders. Adv Mater Res 105–106:213–217. https://doi.org/10.4028/www.scientific.net/AMR.105-106.213
Li F, Cao Y, Liu J et al. (2017) Oxidation resistance of ZrB2 and ZrB2-SiC ultrafine powders synthesized by a combined sol-gel and boro/carbothermal reduction method. Ceram Int 43:7743–7750. https://doi.org/10.1016/j.ceramint.2017.03.080
Wang T, Zhang Y, Li J et al. (2015) Synthesis of ZrB2 and ZrB2-SiC powders using a sucrose-containing system. J Nanosci Nanotechnol 15:7402–7406. https://doi.org/10.1166/jnn.2015.10583
Simonenko EP, Simonenko NP, Papynov EK et al. (2018) Production of HfB2–SiC (10–65 vol % SiC) ultra-high-temperature ceramics by hot pressing of HfB2–(SiO2–C) composite powder synthesized by the sol–gel method. Russ J Inorg Chem 63:1–15. https://doi.org/10.1134/S0036023618010187
Bannykh D, Utkin A, Baklanova N (2018) Effect of chromium additive on sintering and oxidation behavior of HfB2-SiC ceramics. Ceram Int 44:12451–12457. https://doi.org/10.1016/j.ceramint.2018.04.035
Tong Z, He R, Cheng T et al. (2018) High temperature oxidation behavior of ZrB2-SiC added MoSi2 ceramics. Ceram Int 44:21076–21082. https://doi.org/10.1016/j.ceramint.2018.08.144
Grigoriev ON, Panasyuk AD, Podchernyaeva IA et al. (2018) Mechanism of high-temperature oxidation of ZrB2-based composite ceramics in the ZrB2–SiC–AlN system. Powder Metall Met Ceram 57:71–74. https://doi.org/10.1007/s11106-018-9956-2
Vinci A, Zoli L, Sciti D (2018) Influence of SiC content on the oxidation of carbon fibre reinforced ZrB2/SiC composites at 1500 and 1650 °C in air. J Eur Ceram Soc 38:3767–3776. https://doi.org/10.1016/j.jeurceramsoc.2018.04.064
Das J, Kesava BC, Reddy JJ et al. (2018) Microstructure, mechanical properties and oxidation behavior of short carbon fiber reinforced ZrB2-20v/oSiC-2v/oB4C composite. Mater Sci Eng A 719:206–226. https://doi.org/10.1016/j.msea.2018.01.124
Guérineau V, Julian-Jankowiak A (2018) Oxidation mechanisms under water vapour conditions of ZrB2-SiC and HfB2-SiC based materials up to 2400 °C. J Eur Ceram Soc 38:421–432. https://doi.org/10.1016/j.jeurceramsoc.2017.09.015
Mallik M, Ray K, Mitra R (2017) Effect of Si3N4 addition on oxidation resistance of ZrB2-SiC composites. Coatings 7:92. https://doi.org/10.3390/coatings7070092
Vinci A, Zoli L, Landi E, Sciti D (2017) Oxidation behaviour of a continuous carbon fibre reinforced ZrB2–SiC composite. Corros Sci 123:129–138. https://doi.org/10.1016/j.corsci.2017.04.012
Opeka MM, Talmy IG, Zaykoski JA (2004) Oxidation-based materials selection for 2000°C+ hypersonic aerosurfaces: Theoretical considerations and historical experience. J Mater Sci 39:5887–5904. https://doi.org/10.1023/B:JMSC.0000041686.21788.77
Jin H, Meng S, Xinghong Z et al. (2016) Oxidation of ZrB2-SiC-graphite composites under low oxygen partial pressures of 500 and 1500 Pa at 1800°C. J Am Ceram Soc 99:2474–2480. https://doi.org/10.1111/jace.14232
Xie W, Peng Z, Jin H et al. (2018) Fabrication and thermal structural characteristics of ultra-high temperature ceramic struts in scramjets. J Wuhan Univ Technol Sci Ed 33:375–380. https://doi.org/10.1007/s11595-018-1832-9
Parthasarathy TA, Petry MD, Cinibulk MK et al. (2013) Thermal and oxidation response of UHTC leading edge samples exposed to simulated hypersonic flight conditions. J Am Ceram Soc 96:907–915. https://doi.org/10.1111/jace.12180
Cecere A, Savino R, Allouis C, Monteverde F (2015) Heat transfer in ultra-high temperature advanced ceramics under high enthalpy arc-jet conditions. Int J Heat Mass Transf 91:747–755. https://doi.org/10.1016/j.ijheatmasstransfer.2015.08.029
Abdollahi A, Ehsani N, Valefi Z (2018) High temperature ablation-oxidation performance of SiC nanowhisker toughened-SiC/ZrB2-SiC ultra-high temperature multilayer coatings under supersonic flame. J Alloys Compd 745:798–809. https://doi.org/10.1016/j.jallcom.2018.02.234
Inoue R, Arai Y, Kubota Y et al. (2018) Oxidation behavior of carbon fiber-dispersed ZrB2-SiC-ZrC triple phase matrix composites in an oxyhydrogen torch environment. Ceram Int 44:8387–8396. https://doi.org/10.1016/j.ceramint.2018.02.031
Kubota Y, Yano M, Inoue R et al. (2018) Oxidation behavior of ZrB2-SiC-ZrC in oxygen-hydrogen torch environment. J Eur Ceram Soc 38:1095–1102. https://doi.org/10.1016/j.jeurceramsoc.2017.11.024
Monteverde F, Savino R (2012) ZrB2-SiC sharp leading edges in high enthalpy supersonic flows. J Am Ceram Soc 95:2282–2289. https://doi.org/10.1111/j.1551-2916.2012.05226.x
Jin X, He R, Zhang X, Hu P (2013) Ablation behavior of ZrB2–SiC sharp leading edges. J Alloys Compd 566:125–130. https://doi.org/10.1016/j.jallcom.2013.03.067
Simonenko EP, Simonenko NP, Gordeev AN et al. (2018) Impact of a subsonic dissociated air flow on the surface of HfB2–30 vol % SiC UHTC produced by the sol–gel method. Russ J Inorg Chem 63:1345–1355. https://doi.org/10.1134/S0036023618100170
Simonenko EP, Simonenko NP, Gordeev AN et al. (2018) Impact of a supersonic dissociated air flow on the surface of HfB2–30 vol % SiC UHTC produced by the sol–gel method. Russ J Inorg Chem 63:1484–1493. https://doi.org/10.1134/S0036023618110177
Wong-Ng W, Hubbard CR (1987) Standard reference materials for X-ray diffraction Part II. Calibration using d-spacing standards. Powder Diffr 2:242–248. https://doi.org/10.1017/S0885715600012884
Kawamura T (1965) Silicon carbide crystals grown in nitrogen atmosphere. Mineral J 4:333–355. https://doi.org/10.2465/minerj1953.4.333
Holleck H (1967) Legierungsverhalten von HfB2 mit uran- und übergangsmetalldiboriden. J Nucl Mater 21:14–20. https://doi.org/10.1016/0022-3115(67)90724-6
Wyckoff RWG (1963) ZnS structure, sphalerite structure. Cryst Struct 1:85–237
Marschall J, Pejakovic D, Fahrenholtz WG et al. (2012) Temperature jump phenomenon during plasmatron testing of ZrB2-SiC ultrahigh-temperature ceramics. J Thermophys Heat Transf 26:559–572. https://doi.org/10.2514/1.T3798
Ruh R, Corfield PWR (1970) Crystal Structure of monoclinic hafnia and comparison with monoclinic zirconia. J Am Ceram Soc 53:126–129. https://doi.org/10.1111/j.1151-2916.1970.tb12052.x
Kroll P, Milko M (2003) Theoretical investigation of the solid state reaction of silicon nitride and silicon dioxide forming silicon oxynitride (Si2N2O) under pressure. Zeitschrift für Anorg und Allg Chemie 629:1737–1750. https://doi.org/10.1002/zaac.200300122
Aigner K, Lengauer W, Rafaja D, Ettmayer P (1994) Lattice parameters and thermal expansion of Ti(CxN1−x), Zr(CxN1−x), Hf(CxN1−x) and TiN1−x from 298 to 1473 K as investigated by high-temperature X-ray diffraction. J Alloys Compd 215:121–126. https://doi.org/10.1016/0925-8388(94)90828-1
Zaidel’ AN, Prokof’ev VK, Raiskii SM et al. (1969) Tables of spectral lines [in Russ.], Nauka. Moscow
Zaidel’ AN, Prokof’ev VK, Raiskii SM et al. Zaidel tables program package, www.slavna.ru
Acknowledgements
The study has been funded by the Russian Science Foundation (17-73-20181, for obtaining HfB2–SiC ultra-high-temperature ceramic materials with nanocrystalline silicon carbide using the sol–gel process and studying the mechanism of their oxidation), and by the Russian Foundation for Basic Research (No. 17-01-00054-a, for studying the heat transfer of high-enthalpy gas jets with the surface of ceramic samples).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simonenko, E.P., Simonenko, N.P., Gordeev, A.N. et al. Behavior of HfB2–30 vol% SiC UHTC obtained by sol–gel approach in the supersonic airflow. J Sol-Gel Sci Technol 92, 386–397 (2019). https://doi.org/10.1007/s10971-019-05029-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05029-9