Abstract
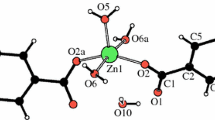
Zn(OAc)2(H2DEA) was synthesized by the reaction of zinc acetate dihydrate (Zn(OAc)2•2H2O) with diethanolamine (H2DEA), and was characterized using single-crystal X-ray structural analysis, nuclear magnetic resonance spectroscopy, Fourier-transform infrared (FT-IR) spectroscopy, and elemental analysis. Zn(OAc)2(H2DEA) had a trigonal bipyramidal geometry comprised of one zinc atom, two acetate groups, and one H2DEA as a neutral tridentate ligand to form two five-membered rings. The states of Zn(OAc)2(H2DEA) heated at various temperatures were determined by FT-IR spectroscopy. At 270 °C, the H2DEA ligand dissociated and was removed. The absorption bands assigned to Zn–O stretching vibration of Zn4O core such as the zinc-oxo cluster appeared. When heated at 500 °C, the absorption bands of μ4-oxozincate and the acetate group disappeared completely and hexagonal wurtzite structural ZnO was formed at 550 °C. A possible thermal decomposition pathway from Zn(OAc)2(H2DEA) to ZnO was proposed. The ZnO film was highly transparent and formed by the deposition of ZnO nanoparticles with size ~40 nm.

Zn(OAc)2(H2DEA) was synthesized and characterized. The states of Zn(OAc)2(H2DEA) heated at various temperatures were determined by FT-IR spectroscopy, and the formation mechanism of ZnO was estimated.
Highlights
-
Zinc–diethanolamine complex was synthesized by the reaction of zinc acetate with diethanolamine.
-
Zinc–diethanolamine complex was isolated and characterized.
-
The formation mechanism of ZnO was estimated by FT-IR spectra.






Similar content being viewed by others
References
Fortunato E, Barquinha P, Martins R (2012) Oxide semiconductor thin-film transistors: a review of recent advances. Adv Mater 24:2945–2986
Tari O, Aronne A, Addonizio ML, Daliento S, Fanelli E, Pernice P (2012) Sol–gel synthesis of ZnO transparent and conductive films: a critical approach. Sol Energy Mater Sol Cells 105:179–186
Sirelkhatim A, Mahmud S, Seeni A, Haida N, Kaus M, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett 7:219–242
Hussain M, Tahir MN, Mansoor MA, Arifin Z, Mazhar M (2013) Heptanuclear zinc cluster for growth of zincite and manganese-doped zincite thin films for sensor applications. Mon Chem 144:285–294
Liu Y, Li Y, Zeng H (2013) ZnO-based transparent conductive thin films: doping, performance, and processing. J Nanomater 2013:196521
Rodnyi PA, Khodyuk IV (2011) Optical and luminescence properties of zinc oxide. Opt Spectrosc 111:776–785
Adl AH, Kar P, Farsinezhad S, Sharma H, Shankar K (2015) Effect of sol stabilizer on the structure and electronic properties of solution-processed ZnO thin films. RSC Adv 5:87007–87018
Stadler A (2012) Transparent conducting oxides—an up-to-date overview. Materials 5:661–683
Kolodziejczk-Radzimska A, Jesionowski T (2014) Zinc oxide—from synthesis to application: a review. Materials 7:2833–2881
Khatibani AB, Abbasi M (2018) Effect of Fe and Co doping on ethanol sensing property of powder-based ZnO nanostructures prepared by the sol–gel method. J Sol–Gel Sci Technol 86:255–265
Li H, Wang J, Liu H, Yang C, Xu H, Li X, Cui H (2004) Sol–gel preparation of transparent zinc oxide films with highly preferential crystal orientation. Vacuum 77:57–62
Znaidi L, GJAAS Illia, Benyahia S, Sanchez C, Kanaev AV (2003) Oriented ZnO thin films synthesis by sol–gel process for laser application. Thin Solid Films 428:257–262
Znaidi L, GJAAS Illia, Guennic RL, Sanchez C, Kanaev A (2003) Elaboration of ZnO thin films with preferential orientation by a soft chemistry route. J Sol–Gel Sci Technol 26:817–821
Chen WJ, Liu WL, Hsieh SH, Hsu YG (2012) Synthesis of ZnO:Al transparent conductive thin films using the sol–gel method. Proc Eng 36:54–61
Salam S, Islam M, Akram A (2013) Sol–gel synthesis of intrinsic and aluminum-doped zinc oxide thin films as transparent conducting oxides for thin film solar cells. Thin Solid Films 529:242–247
Ohya Y, Saiki H, Takahashi Y (1994) Preparation of transparent, electrically conducting ZnO film from zinc acetate and alkoxide. J Mater Sci 29:4099–4103
Znaidi L (2010) Sol–gel-deposited ZnO thin films: a review. Mat Sci Eng B-Solid 174:18–30
Nehmann JB, Ehrmann N, Reineke-Koch R, Bahnemann DW (2014) Aluminum-doped zinc oxide sol–gel thin films: influence of the sol’s water content on the resistivity. Thin Solid Films 556:168–173
Conterosito E, Croce G, Palin L, Boccaleri E, van Beekab W, Milanesio M (2012) Crystal structure and solid-state transformations of Zn–triethanolamine–acetate complexes to ZnO. Cryst Eng Comm 14:4472–4477
Gordon RM, Silver HB (1982) Preparation and properties of tetrazinc μ4-oxohexa-μ-carboxylates (basic zinc carboxylates). Can J Chem 61:1218–1221
Sheldrick GM (1996) SADABS, Program for Simens area detector absorption correction, University of Göttingen, Germany
Sheldrick GM (1997) SHELXS-97, Program for crystal structure solution, University of Göttingen, Germany
Sheldrick GM (1997) SHELXL-97, Program for crystal structure refinement, University of Göttingen, Germany
Johnson MK, Powell DB, Cannon RD (1981) Vibrational spectra of carboxylato complexes—I. Infrared and Raman spectra of beryllium(II) acetate and formate and of zinc(II) acetate and zinc(II) acetate dihydrate. Spectrochim Acta A Mol Spectrosc 37:899–904
Zhang Y, Zhu F, Zhang J, Xia L (2008) Converting layered zinc acetate nanobelts to one-dimensional structured ZnO nanoparticle aggregates and their photocatalytic activity. Nanoscale Res Lett 3:201–204
Masoud MS, El-Enein SAA, Abed IM, Ali AE (2002) Synthesis and characterization of aminoalcohol complexes. J Coord Chem 55:153–178
Brannon DG, Morrison RH, Hall JL, Humphrey GL, Zimmerman DN (1971) Spectra and bonding for copper(II)-aminoalcohol complexes—I: the I.R. spectra of complexes of mono-, di- and triethanolamine. J Inorg Nucl Chem 33:981–990
Berkesi O, Dreveni I, Andor JA, Goggin PL (1991) Formation and mid-FT-IR investigation of short (C2–C5) straight chain tetrazinc μ4-oxohexa-μ-carboxylates. Inorg Chim Acta 181:285–289
Spanhel L (2006) Colloidal ZnO nanostructures and functional coatings: a survey. J Sol–Gel Sci Technol 39:7–24
Meulenkamp EA (1998) Synthesis and growth of ZnO nanoparticles. J Phys Chem B 102:5566–5572
Tokumoto MS, Pulcinelli SH, Santilli CV, Briois V (2003) Catalysis and temperature dependence on the formation of ZnO nanoparticles and of zinc acetate derivatives prepared by the sol–gel route. J Phys Chem B 107:568–574
Schmidt T, Müller G, Spanhel L (1998) Activation of 1.54 μm Er3+ fluorescence in concentrated II–VI semiconductor cluster environments. Chem Mater 10:65–71
Viart N, Richard-Plouet M, Muller D, Pourroy G (2003) Synthesis and characterization of Co/ZnO nanocomposites: toward new perspectives offered by metal/piezoelectric composite materials. Thin Solid Films 437:1–9
O’Brien S, Nolan MG, Çopuroglu M, Hamilton JA, Povey I, Pereira L, Martins R, Fortunato E, Pemble M (2010) Zinc oxide thin films: characterization and potential applications. Thin Solid Films 518:4515–4519
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks” (No. 2401) (JSPS KAKENHI Grant Number JP24102008). This work was also supported by JSPS KAKENHI Grant Number JP16K17951.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hayami, R., Endo, N., Abe, T. et al. Zinc–diethanolamine complex: synthesis, characterization, and formation mechanism of zinc oxide via thermal decomposition. J Sol-Gel Sci Technol 87, 743–748 (2018). https://doi.org/10.1007/s10971-018-4768-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4768-x