Abstract
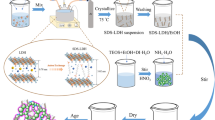
Trimethylethoxysilane (TMES) has been recognized as a good co-precursor to increase the degree of hydrophobicity during the synthesis of a silica aerogel because of its methyl groups. Therefore, some physical properties of silica aerogels, including the contact angle and porosity, were investigated using TMES as a co-precursor at different molar ratios with the main precursor such as tetramethoxysilane (TMOS) or tetraethoxysilane (TEOS). In contrast to TMES, most silylating agents such as hexamethyldisilazane (HMDZ) and trimethylchlorosilane (TMCS) have been used for surface modification because of their ability to enhance the hydrophobicity of the aerogel surface. This work examines the silylation effect, which includes increasing hydrophobicity by TMES to determine the possibility of using it as an alternative silylating agent during ambient pressure drying in the synthesis of sodium silicate-based silica aerogel. In addition, the physical properties of sodium silicate-based silica aerogels with silylation under different TMES/TMCS volume ratio are investigated. The physical properties of sodium silicate-based aerogels can be changed by the TMES/TMCS volume ratio during the surface modification step. Aerogels with a high specific surface area (458 m2/g), pore volume (3.215 cm3/g), porosity (92.7%), and contact angle (131.8°) can be obtained TMES/TMCS volume ratio of 40/60.

Highlights
-
Sodium silicate-based silica aerogel was synthesized by APD using TMES/TMCS surface co-modifying agent.
-
TMES can act as an alternative surface modification agent with TMCS.
-
Hydrophobicity was not measured at the case of only TMES usage.
-
TMES/TMCS surface co-modifier for silylation enhances the physical properties of silica aerogel.












Similar content being viewed by others
References
Parale VG, Mahadik DB, Kavale MS, Rao AV, Wagh PV, Gupta SC (2011) Potential application of silica aerogel granules for cleanup of accidental spillage of various organic liquids. Soft Nanosci Lett 1:97–104
Dorcheh AS, Abbasi MH (2008) Silica aerogel; synthesis, properties and characterization. J Mater Process Technol 199(1-3):10–26
Schmidt M, Schwertfeger F (1998) Applications for silica aerogel products. J Non-Cryst Solids 225:364–368
Parale VG, Jung H-N-R, Han W, Lee KY, Mahadik DB, Cho HH, Park HH (2017) Improvement in the high temperature thermal insulation performance of Y2O3 opacified silica aerogels. J Alloy Comp 727:871–878
Parale VG, Lee KY, Park HH (2017) Flexible and transparent silica aerogels: An overview. J Korean Ceram Soc 54(3):184–199
Schwertfeger F, Frank D, Schmidt M (1998) Hydrophobic waterglass based aerogels without solvent exchange or supercritical drying. J Non-Cryst Solids 225:24–29
Lee CJ, Kim GS, Hyun SH (2002) Synthesis of silica aerogels from waterglass via new modified ambient drying. J Mater Sci 37:2237–2241
Bhagat SD, Kim Y-H (2007) A cost-effective and fast synthesis of nanoporous SiO2 aerogel powders using water-glass via ambient pressure drying route. Solid State Sci 9:628–635
Prakash SS, Brinker CJ, Hurd AJ, Rao SM (1995) Silica aerogel films prepared at ambient pressure by using surface derivatization to induce reversible drying shrinkage. Nature 374(30):439–443
Shi F, Wang L, Liu J (2006) Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater Lett 60(29-30):3718–3722
Kim GS, Hyun SH (2003) Synthesis of window glazing coated with silica aerogel films via ambient drying. J Non-Cryst Solids 320(1-3):125–132
Mahadik SA, Pedraza F, Parale VG, Park HH (2016) Organically modified silica aerogel with different functional silylating agents and effect on their physico-chemical properties. J Non-Cryst Solids 453:164–171
Shewale PM, Rao AV (2008) Effect of different trimethyl silylating agents on the hydrophobic and physical properties of silica aerogels. Appl Surf Sci 254:6902–6907
Gurav JL, Rao AV, Rao AP, Nadargi DY, Bhagat SD (2009) Physical properties of sodium silicate based silica aerogels prepared by single step sol–gel process dried at ambient pressure. J Alloy Comp 476:397–402
Rao AP, Rao AV (2010) Modifying the surface energy and hydrophobicity of the low-density silica aerogels through the use of combinations of surface-modification agents. J Mater Sci 45(1):51–63
Rao AP, Rao AV (2009) Improvement in optical transmission of the ambient pressure dried hydrophobic nanostructured silica aerogels with mixed silylating agents. J Non-Cryst Solids 355:2260–2271
Rao AV, Kulkarni MM (2003) Synthesis and characterization of hydrophobic silica aerogels using trimethylethoxysilane as a co-precursor. J Sol-Gel Sci Technol 27:103–109
Rao AV, Kulesh RR, Amalnerkar DP, Seth T (2003) Synthesis and characterization of hydrophobic TMES/TEOS based silica aerogels. J Porous Mater 10:23–29
Hegde N, Hirashima H, Rao AV (2007) Two step sol-gel processing of TEOS based hydrophobic silica aerogels using trimethylethoxysilane as a co-precursor. J Porous Mater 14:165–171
Latthe SS, Imai H, Ganesan V, Rao AV (2009) Superhydrophobic silica films by sol–gel co-precursor method. Appl Surf Sci 256:217–222
Nah H-Y, Jung H-N-R, Lee K-Y, Ku YS, Park H-H (2017) Effect of acid catalyst kinds on the pore structural characteristics of water glass based silica aerogel. J Microelectron Packag Soc 24(3):13–18
Bangi UKH, Jung IK, Park CS, Baek S, Park HH (2013) Optically transparent silica aerogels based on sodium silicate by a two step sol–gel process and ambient pressure drying. Solid State Sci 18:50–57
Yu Y, Guo D, Fang J (2015) Synthesis of silica aerogel microspheres by a two-step acid–base sol–gel reaction with emulsification technique. J Porous Mater 22(3):621–628
Dervin S, Lang Y, Perova T, Hinder SH, Pillai SC (2017) Graphene oxide reinforced high surface area silica aerogels. J Non-Cryst Solids 465:31–38
Nah H-Y, Parale VG, Jung H-N-R, Lee K-Y, Lim C-H, Ku YS, Park H-H (2018) Role of oxalic acid in structural formation of sodium silicate-based silica aerogel by ambient pressure drying. J Sol-Gel Sci Technol 85:302–310
Hwang SW, Kim TY, Hyun SH (2008) Optimization of instantaneous solvent exchange/surface modification process for ambient synthesis of monolithic silica aerogels. J Colloid Interface Sci 322:224–230
Parale VG, Mahadik DB, Mahadik SA, Kavale MS, Rao AV, Wagh PB (2012) Wettability study of surface modified silica aerogels with different silylating agents. J Sol-Gel Sci Technol 63(3):573–579
Parale VG, Mahadik DB, Kavale MS, Mahadik SA, Rao AV, Mullens S (2013) Sol–gel preparation of PTMS modified hydrophobic and transparent silica coatings. J Porous Mater 20:733–739
Rao AP, Pajonk GM, Rao AV (2005) Effect of preparation conditions on the physical and hydrophobic properties of two step processed ambient pressure dried silica aerogels. J Mater Sci 40(13):3481–3489
Jal PK, Patel S, Mishra BK (2004) Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 62(5):1005–1028
Belyakova LA, Varvarin AM (1999) Surfaces properties of silica gels modified with hydrophobic groups. Colloids Surf A Physicochem Eng Asp 154(3):285–294
Bhagat SD, Kim YH, Suh KH, Ahn YS, Yeo JG, Han JH (2008) Superhydrophobic silica aerogel powders with simultaneous surface modification, solvent exchange and sodium ion removal from hydrogels. Microporous Mesoporous Mater 112(1-3):504–509
Zhou D, Seraphin S (1994) Production of silicon carbide whiskers from carbon nanoclusters. Chem Phys Lett 222:233–238
Wang LJ, Zhao SY, Yang M (2009) Structural characteristics and thermal conductivity of ambient pressure dried silica aerogels with one-step solvent exchange/surface modification. Mater Chem Phys 113:485–490
Mahadik DB, Rao AV, Rao AP, Wagh PB, Ingale SV, Gupta SC (2011) Effect of concentration of trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDZ) silylating agents on surface free energy of silica aerogels. J Colloid Interface Sci 356:298–302
Yokogawa H, Yokoyama M (1995) Hydrophobic silica aerogels. J Non-Cryst Solids 186:23–29
Sarawade PB, Kim JK, Hilonga A, Kim HT (2010) Production of low-density sodium silicate-based hydrophobic silica aerogel beads by a novel fast gelation process and ambient pressure drying process. Solid State Sci 12:911–918
Bhagat SD, Kim YH, Ahn YS, Yeo JG (2007) Rapid synthesis of water-glass based aerogels by in situ surface modification of the hydrogels. Appl Surf Sci 253:3231–3236
Wu G, Yu Y, Cheng X, Zhang Y (2011) Preparation and surface modification mechanism of silica aerogels via ambient pressure drying. Mater Chem Phys 129:308–314
Einarsrud M-A, Nilsen E (1998) Strengthening of water glass and colloidal sol based silica gels by aging in TEOS. J Non-Cryst Solids 226:122–128
Bangi UKH, Rao AV, Rao AP (2008) A new route for preparation of sodium-silicate-based hydrophobic silica aerogels via ambient-pressure drying. Sci Technol Adv Mater 9:1–10
Rao AP, Rao AV, Pajonk GM (2007) Hydrophobic and physical properties of the ambient pressure dried silica aerogels with sodium silicate precursor using various surface modification agents. Appl Surf Sci 253(14):6032–6040
Rao AV, Kulkarni MM (2002) Effect of glycerol additive on physical properties of hydrophobic silica aerogels. Mater Chem Phys 77(3):819–825
Acknowledgements
This work was supported by Nano-Convergence Foundation (www.nanotech2020.org) funded by the Ministry of Science and ICT (MSIT, Korea) & the Ministry of Trade, Industry and Energy (MOTIE, Korea) [Project Name: Commercialization development of super thermal insulation aerogel composite foam for cold insulation material]. This work was supported by the Center for Advanced Meta-Materials (CAMM) funded by the Ministry of Science, ICT and Future Planning as Global Frontier Project (CAMM-No. NRF-2014M3A6B3063716).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Nah, HY., Parale, V.G., Lee, KY. et al. Silylation of sodium silicate-based silica aerogel using trimethylethoxysilane as alternative surface modification agent. J Sol-Gel Sci Technol 87, 319–330 (2018). https://doi.org/10.1007/s10971-018-4729-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4729-4