Abstract
Superhydrophilic surfaces without the need of other stimuli are usually realized by constructing a rough morphology. However, constructing rough surfaces usually require specialized equipment or complicated processing. Besides, rough surfaces can cause undesirable scattering, which strongly limits the use in optical devices. In this article, we prepared superhydrophilic TiO2 films with ultra-smooth surfaces using simple sol-gel dip-coating method. The hydrophilicity of the TiO2 films varied with different post-heat treatments. The films heat-treated at 400 °C exhibited a durable superhydrophilicity and anti-fogging property. This superhydrophilicity was attributed to the decrease of surface hydrophobic alkoxy groups and the formation of point defects, i.e., Ti3+ and oxygen vacancies, which are favourable for dissociative water adsorption. The amount of surface organic groups was influenced by autophobicity effects, further hydrolysis and decomposition of residual alkoxy groups. Additionally, the wettability behaviours of the films were also explained from the perspective of the surface energy. These results can benefit the design and manufacture of anti-fogging and self-cleaning superhydrophilic TiO2 films.

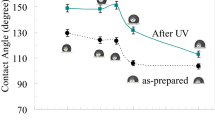
The TiO2 films exhibited intrinsic superhydrophilicity and anti-fogging property; the superhydrophilicity can maintain 30 days.
Highlights
-
The TiO2 films exhibited durability, superhydrophilicity and anti-fogging property.
-
The superhydrophilicity films with smooth surface were capable of being optical materials.
-
The mechanism of the superhydrophilicity was well studied.









Similar content being viewed by others
References
Saxena N, Naik T, Paria S (2017) J Phys Chem C 121:2428–2436
Shi H, He Y, Pan Y, Di H, Zeng G, Zhang L, Zhang C (2016) J Memb Sci 506:60–70
Faustini M, Grenier A, Naudin G, Li R, Grosso D (2015) Nanoscale 7:19419–17425
Fujima T, Futakuchi E, Tomita T, Orai Y, Sunaoshi T (2014) Langmuir 30:14494–14497
Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T (1997) Nature 388:431–432
Sakai N, Fujishima A, Watanabe T, Hashimoto K (2003) J Phys Chem B 107:1028–1035
Fujishima A, Zhang X, Tryk D (2008) Surf Sci Rep 63:515–582
Park JJ, Kim DY, Latthe SS, Lee JG, Swihart MT, Yoon SS (2013) ACS Appl Mater Interfaces 5:6155–6160
Diebold U (2003) Surf Sci Rep 48:53–229
Bharti B, Kumar S, Kumar R (2016) Appl Surf Sci 364:51–60
Yao L, He J (2014) J Mater Chem A 2:6994–7003
Wenzel RN (1949) J Phys Colloid Chem 53:1466–1467
Song S, Jing L, Li S, Fu H, Luan Y (2008) Mater Lett 62:3503–3505
Wang J, Wang JJ, Sun Y-L, Wang C-W (2013) J Sol-Gel Sci Technol 68:75–80
Huang W, Chen Y, Yang C, Situ Y, Huang H (2015) Ceram Int 41:7573–7581
Kumar R, Singh RK, Kumar M, Barthwal SK (2007) J Appl Polym Sci 104:767–772
Ebert J, Pannhorst H, Küster H, Welling H (1979) Appl Opt 18:818–822
Xiong Y, Lai M, Li J, Yong H, Qian H, Xu C, Zhong K, Xiao S (2015) Surf Coat Technol 265:78–82
Ashkarran AA, Mohammadizadeh MR (2008) Mater Res Bull 43:522–530
Zhang H, Liu Y, Wu Y, Ruan K (2015) J Nanosci Nanotechnol 15:2531–2536
Livage J, Henry M, Sanchez C (1988) Progress Solid State Chem 18:259–341
Vold MJ (1963) J Colloid Sci 18:684–695
Tan SN, Zeng X, Fokkink B (2013) Surf Eng 16:235–238
Vargas MA, Rodríguez-Páez JE (2017) J Non-Cryst Solids 459:192–205
Li X, He J (2013) ACS Appl Mater Interfaces 5:5282–5290
Di Mundo R, d’Agostino R, Palumbo F (2014) ACS Appl Mater Interfaces 6:17059–17066
Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T (1998) Adv Mater 10:135–138
Lu GQ, Linsebigler A, Yates JT (1994) J Phys Chem 98:11733–11738
Henderson MA (1996) Surf Sci 355:151–166
Kumar R, Govindarajan S, Siri Kiran Janardhana RK, Rao TN, Joshi SV, Anandan S (2016) ACS Appl Mater Interfaces 8:27642–27653
Song Z, Hrbek J, Osgood R (2005) Nano Lett 5:1327–1332
Yu J, Zhao X, Zhao Q (2000) Thin Solid Films 379:7–14
Pouilleau J, Devilliers D, Groult H, Marcus P (1997) J Mater Sci 32:5645–5651
Dupin J-C, Gonbeau D, Vinatier P, Levasseur A (2000) Phys Chem Chem Phys 2:1319–1324
Naldoni A, Allieta M, Santangelo S, Marelli M, Fabbri F, Cappelli S, Bianchi CL, Psaro R, Dal Santo V (2012) J Am Chem Soc 134:7600–7603
Zuo F, Wang L, Wu T, Zhang Z, Borchardt D, Feng P (2010) J Am Chem Soc 132:11856–11857
Tauc J, Grigorovici R, Vancu A (1966) Phys Status Solidi 15:627–631
Tanemura S, Miao L, Jin P, Kaneko K, Terai A, Nabatova-Gabain N (2003) Appl Surf Sci 212–213:654–660
Justicia I, Ordejon P, Canto G, Mozos JL, Fraxedas J, Battiston GA, Gerbasi R, Figueras A (2002) Adv Mater 14:1399–1402
Owens DK, Wendt RC (1969) J Appl Polym Sci 13:1741–1748
Kong F, Xia Y, Jiao X, Chen D (2017) New J Chem 41:7562–7570
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, Y., Xia, B. & Jiang, B. Thermal-induced durable superhydrophilicity of TiO2 films with ultra-smooth surfaces. J Sol-Gel Sci Technol 87, 50–58 (2018). https://doi.org/10.1007/s10971-018-4684-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4684-0