Abstract
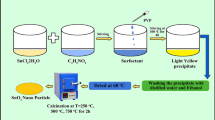
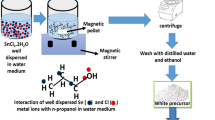
Precipitation is one of the dominant techniques for producing tin oxide which requires calcination of the interim tin oxide hydroxide (Sn6O4(OH)4) precipitate at different temperatures to render the requisite oxide states. In this study, we mitigated the necessity for calcination through simple ageing of the the as-synthesized Sn6O4(OH)4 at room temperature (25 °C) in aqueous medium. Ageing for 12 days resulted in phase transition of the interim precipitate and production of discernible layers of black stannous oxide (SnO) and yellow stannic oxide (SnO2) along with the originally formed white layer of Sn6O4(OH)4 within the same aqueous medium. The phases of tin oxide was confirmed after extracting individual layer and assessing them through X-ray analysis. Thermogravimetric analysis for the as-obtained stannous oxide established its thermal stability up to around 410 °C. Additionally, Field Emission Scanning Electron Microscopy studies performed on the SnO nanoparticles revealed their dimension to be within 25–30 nm range. The mechanisms for SnO and SnO2 formation through ageing has been delineated.
Graphical Abstract




Similar content being viewed by others
References
Lee HN, Song BJ, Park JC (2014) Fabrication of p-channel amorphous tin oxide thin-film transistors using a thermal evaporation process. J Disp Technol 10:288–292
Zhao P, Sun H, Tian L, Wang B, Liu F, Sun P, Lu G (2015) Interfacial engineering and configuration design of bilayered photoanode consisting of macroporous tin dioxide/titanium dioxide for high performance dye-sensitized solar cells. Electrochim Acta 176:845–852
Ghosh S, Narjinary M, Sen A, Bandyopadhyay R, Roy S (2014) Fast detection of low concentration carbon monoxide using calcium-loaded tin oxide sensors. Sens Actuators B: Chem 203:490–496
Peters K, Zeller P, Stefanic G, Skoromets V, Nemec H, Kuzel P, Fattakhova-Rohlfing D (2015) Water-dispersible small monodisperse electrically conducting antimony doped tin oxide nanoparticles. Chem Mater 27:1090–1099
Kandpal S, Saxena AK (2015) Synthesis & characterization of novel silicone dendrimers as base stock for high performance lubricants. J Organomet Chem 791:232–237
Yin L, Chai S, Wang F, Huang J, Li J, Liu C, Kong X (2016) Ultrafine SnO2 nanoparticles as a high performance anode material for lithium ion battery. Ceram Int 42:9433–9437
Nayak PK, Caraveo-Frescas JA, Wang Z, Hedhili MN, Wang QX, Alshareef NH (2014) Thin film complementary metal oxide semiconductor (CMOS) device using a single-step deposition of the channel layer. Sci Rep 4(1-7):4672
Lu P, Hu X, Huang H, Hu N, Zhang J, Shen X (2016) Controllable low-temperature hydrothermal synthesis and gas-sensing investigation of crystalline SnO2 nanoparticles. J Mater Eng Perform 25:1342–1346
Zhu Y, Guo H, Zhai H, Cao C (2015) Microwave-assisted and gram-scale synthesis of ultrathin SnO2 nanosheets with enhanced lithium storage properties. ACS Appl Mater Interfaces 7:2745–2753
Majumder S (2015) The effects of crystallite size, surface area and morphology on the sensing properties of nanocrystalline SnO2 based system. Ceram Int 41:14350–14358
Dang TV, Hoa ND, Duy NV, Hieu NV (2016) Chlorine gas sensing performance of on-chip grown ZnO, WO3, and SnO2 nanowire sensors. ACS Appl Mater Interfaces 8:4828–4837
Jeong S, McDowell MT, Cui Y (2011) Low-temperature self-catalytic growth of tin oxide nanocones over large areas. ACS Nano 5:5800–5807
Partington JR, Moser JW (1944) Red stannous oxide. Nature 154:643–643
Moreno MS, Punte G, Rigotti G, Mercader RC, Weisz AD, Blesa MA (2001) Kinetic study of the disproportionation of tin monoxide. Solid State Ion 144:81–86
Wang H, Wang Y, Xu J, Yang H, Lee CS, Rogach AL (2012) Polyvinylpyrrolidone-assisted ultrasonic synthesis of SnO nanosheets and their use as conformal templates for tin dioxide nanostructures. Langmuir 28:10597–10601
Saito G, Hosokai S, Tsubota M, Akiyama T (2012) Influence of solution temperature and surfactants on morphologies of tin oxide produced using a solution plasma technique. Cryst Growth Des 12:2455–2459
Uchiyama H, Imai H (2007) Tin oxide meshes consisting of nanoribbons prepared through an intermediate phase in an aqueous solution. Cryst Growth Des 7:841–843
Uchiyama H, Ohgi H, Imai H (2006) Selective preparation of SnO2 and SnO crystals with controlled morphologies in an aqueous solution system. Cryst Growth Des 6:2186–2190
Moon CS, Kim HR, Auchterlonie G, Drennan J, Lee JH (2008) Highly sensitive and fast responding CO sensor using SnO2 nanosheets. Sens Actuators B: Chem 131:556–564
Ibarguen CA, Mosquera A, Parra R, Castro MS, Rodríguez-Páez JE (2007) Synthesis of SnO2 nanoparticles through the controlled precipitation route. Mater Chem Phys 101:433–440
Song P, Wen D (2009) Experimental investigation of the oxidation of tin nanoparticles. J Phys Chem C 113:13470–13476
Ning J, Dai Q, Jiang T, Men K, Liu D, Xiao N, Li C, Li D, Liu B, Zou B, Zou G, Yu WW (2009) Facile synthesis of tin oxide nanoflowers: a potential high-capacity lithium-ion-storage material. Langmuir 25:1818–1821
Acknowledgments
The present research was funded by Council of Scientific and Industrial Research (CSIR), Government of India, through the 12th 5 year plan network project ‘MULTIFUN’ (Grant number: CSC-0101). The authors have also utilized the facilities of the DST-CSIR Sensor Hub, Kolkata.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10971-017-4381-4.
Rights and permissions
About this article
Cite this article
Ghosh, S., Roy, S. Effect of ageing on Sn6O4(OH)4 in aqueous medium—simultaneous production of SnO and SnO2 nanoparticles at room temperature. J Sol-Gel Sci Technol 81, 769–773 (2017). https://doi.org/10.1007/s10971-016-4251-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4251-5