Abstract
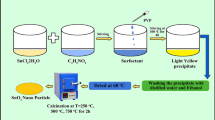
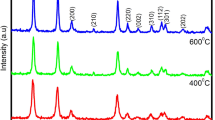
Nanocrystalline phase pure Tin Oxide (SnO2) materials were prepared by chemical co-precipitation method. The materials were prepared using water and 2-propanol as the co-solvents taken in 1:1 ratio. The role of varying the molar concentrations of the raw material and the effect of calcination temperature on the structural, optical, and the electrochemical properties of the SnO2 were explored. The stoichiometric ratio of Tin: Oxygen was found to be 1:2 in all the cases as observed from the EDAX studies. Irrespective of the calcination temperature, the SnO2 made using the 0.4 M of Tin source outperformed other materials in terms of electrochemical performance owing to the good synergy between structural and optical properties. The above material calcinated at 800 °C exhibited an outstanding specific capacitance of 580 F/g at a current density of 1 A/g. Electrochemical impedance spectroscopy indicates the capacitive behavior of the material.














Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
F. Boran, S. Cetinkaya, M.E. Sahin, Effect of surfactant types on the size of tin oxide nanoparticles. Acta Phys. Pol., A 132, 546–548 (2017)
A. Sharma, A. Ahmed, A. Singh, S.K. Oruganti, A. Khosla, S. Arya, Recent advances in tin oxide nanomaterials as electrochemical/chemiresistive sensors. J. Electrochem. Soc. 168(2), 027505 (2021)
M.M. Bagheri-Mohagheghi, N. Shahtahmasebi, M.R. Alinejad, A. Youssefi, M. Shokooh-Saremi, The effect of the post-annealing temperature on the nano-structure and energy band gap of SnO2 semiconducting oxide nano-particles synthesized by polymerizing–complexing sol–gel method. Physica B 403(13–16), 2431–2437 (2008)
S. Das, V. Jayaraman, SnO2: a comprehensive review on structures and gas sensors. Prog. Mater. Sci. 66, 112–255 (2014)
P.S. Subbarao, Y. Aparna, K.L. Chitturi, Synthesis and characterization of Ni doped SnO2 nanoparticles by sol-gel method for novel applications. Mater. Today: Proc. 26, 1676–1680 (2020)
Q. Zhao, L. Ma, Q. Zhang, C. Wang, X. Xu, SnO2-based nanomaterials: synthesis and application in lithium-ion batteries and supercapacitors. J. Nanomater. 850147, 1–15 (2015)
G.E. Patil, D.D. Kajale, V.B. Gaikwad, G.H. Jain, Spray pyrolysis deposition of nanostructured tin oxide thin films, International Scholarly Research. Network 275872, 1–5 (2012)
H.X. Yang, J.F. Qian, Z.X. Chen, X.P. Ai, Y.L. Cao, Multilayered nanocrystalline SnO2 hollow microspheres synthesized by chemically induced self-assembly in the hydrothermal environment. J. Phys. Chem. C 111(38), 14067–14071 (2007)
Z. Chen, J.K.L. Lai, C.H. Shek, H. Chen, Synthesis and structural characterization of rutile SnO2 nanocrystals. J. Mater. Res. 18(06), 1289–1292 (2003)
M. Aziz, S. Saber Abbas, W.R. Wanb Aharom, Size-controlled synthesis of SnO2 nanoparticles by sol–gel method. Mater. Lett. 91, 31–34 (2013)
R. Bargougui, K. Omri, A. Mhemdi, S. Ammar, Synthesis and characterization of SnO2 nanoparticles: effect of hydrolysis rate on the optical properties. Adv. Mater. Lett. 6(9), 816–819 (2015)
A. Hassanzadeh, B. Moazzez, H. Haghgooie, M. Nasseri, M. Golzan, H. Sedghi, Synthesis of SnO2 nanopowders by a sol-gel process using propanol-isopropanol mixture. Open Chem. 6(4), 651–656 (2008)
R.N. Mariammal, N. Rajamanickam, K. Ramachandran, Synthesis and characterization of undoped and co-doped SnO2 nanoparticles. J. Nano Electron. Phys. 3(1), 92–100 (2011)
F. Gu, S.F. Wang, M.K. Lu, G.J. Zhou, D. Xu, D.R. Yuan, Photoluminescence properties of SnO2 nanoparticles synthesized by sol−gel method. J. Phys. Chem. B 108(24), 8119–8123 (2004)
P. Muhammed Shafi, A. Chandrabose, Impact of crystalline defects and size on Xray line broadening: a phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 5, 057137 (2015)
N. Rani, N. Jaggi, Effect of reaction temperature on the structural and electronic properties of stannic oxide nanostructures. Bull. Mater. Sci. 43, 146 (2020)
G. Zhang, X. Xiao, B. Li, P. Gu, H. Xue, H. Pang, Transition metal oxides with one-dimensional/one-dimensional-analogue nanostructures for advanced supercapacitors. J. Mater. Chem. A 5, 8155–8186 (2017)
P. Khaenamkaew, D. Manop, C. Tanghengjaroen, W. Palakawong Na Ayuthaya, Crystal structure, lattice strain, morphology, and electrical properties of SnO2 nanoparticles induced by low calcination temperature. Adv. Mater. Sci. Eng. 3852421, 1–10 (2020)
M. Scimeca, S. Bischetti, H.K. Lamsira, R. Bonfiglio, E. Bonanno, Energy Dispersive X-ray (EDX) microanalysis: a powerful tool in biomedical research and diagnosis. Eur. J. Histochem. 62(1), 2841 (2018)
A. Azam, A.S. Ahmed, S.S. Habib, A.H. Naqvi, Effect of Mn doping on the structural and optical properties of SnO2 nanoparticles. J. Alloy. Compd. 523, 83–87 (2012)
A. Bhattacharjee, M. Ahmaruzzaman, T. Sinha, A novel approach for the synthesis of SnO2 nanoparticles and its application as a catalyst in the reduction and photodegradation of organic compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 136, 751–760 (2015)
S. Naz, I. Javid, S. Konwar, K. Surana, P.K. Singh, M. Sahni, B. Bhattacharya, A simple low cost method for synthesis of SnO2 nanoparticles and its characterization. SN Appl. Sci. 2, 975 (2020)
K. Sujatha, T. Seethalakshmi, A.P. Sudha, O.L. Shanmugasundaram, Photoluminescence properties of pure, Fe-doped and surfactant-assisted Fe-doped tin-oxide nanoparticles. Bull. Mater. Sci. 43, 1–10 (2020)
J. Zhang, L. Gao, Synthesis and characterization of nanocrystalline tin oxide by sol–gel method. J. Solid State Chem. 177(4–5), 1425–1430 (2004)
L. Tan, L. Wang, Y. Wang, Hydrothermal synthesis of SnO2 nanostructures with different morphologies and their optical properties. J. Nanomater. 529874, 1–10 (2011)
B. Saravanakumar, S.P. Ramachandran, G. Ravi, V. Ganesh, S. Ravichandran, P. Muthu Mareeswaran, R. Yuvakkumar, Enhanced pseudocapacitive performance of SnO2, Zn-SnO2, and Ag-SnO2 nanoparticles. Ionics 24(12), 2727 (2018)
K. Rafique, M.Z.U. Shah, A. Shah, M.S.U. Shah, H. Hou, M. Sajjad, M. Arif, S.A. Ahmad, N. Hassan, Electrochemical performance evaluation of a newly developed ZnS–SnO2 composite in an aqueous electrolyte. J. Mater. Sci. Mater. Electron. 34(24), 1717 (2023)
V. Velmurugan, U. Srinivasarao, R. Ramachandran, M. Saranya, A.N. Grace, Synthesis of tin oxide/graphene (SnO2/G) nanocomposite and its electrochemical properties for supercapacitor applications. Mater. Res. Bull. 84, 145–151 (2016)
S. Alkhalaf, C.K. Ranaweera, P.K. Kahol, K. Siam, H. Adhikari, S.R. Mishra, F. Perez, B.K. Gupta, K. Ramasamy, R.K. Gupta, Electrochemical energy storage performance of electrospun CoMn2O4 nanofibers. J. Alloy. Compd. 692, 59–66 (2017)
V. Gowthambabu, S.S. Kanmani, N. Rajamanickam, Influence of anionic precursors on electrochemical properties of tin oxide nanoparticles: a comparative analysis. J. Mater. Sci. Mater. Electron. 9, 11695–11708 (2021)
S. Suthakaran, S. Dhanapandian, N. Krishnakumar, N. Ponpandian, Hydrothermal synthesis of surfactant assisted Zn doped SnO2 nanoparticles with enhanced photocatalytic performance and energy storage performance. J. Phys. Chem. Solids 141, 109407 (2020)
D. Dodoo-Arhin, R.A. Nuamah, P.K. Jain, D.O. Obada, A. Yaya, Nanostructured stannic oxide: synthesis and characterisation for potential energy storage applications. Results Phys. 9, 1391–1402 (2018)
N. Elgrishi, K.J. Rountree, B.D. McCarthy, E.S. Rountree, T.T. Eisenhart, J.L. Dempsey, A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95(2), 197–206 (2018)
B. Varshney, M.J. Siddiqui, A.H. Anwer, M.Z. Khan, F. Ahmed, A. Aljaafari, H.H. Hammud, A. Azam, Synthesis of mesoporous SnO2/NiO nanocomposite using modified sol–gel method and its electrochemical performance as electrode material for supercapacitors. Sci. Rep. 10(1), 1–13 (2020)
H. Cui, Y. Liu, W. Ren, M. Wang, Y. Zhao, Large scale synthesis of highly crystallized SnO2 quantum dots at room temperature and their high electrochemical performance. Nanotechnology 24(34), 345602 (2013)
M. Imran, X. Zhang, Recent developments on the cyclic stability in elastocaloric materials. Mater. Design 195, 109030 (2020)
S. Hashemizadeh, M. Ahmadi, R. Gholipur, Ni0.85Co0.15MoO electrodes for supercapacitors with good cycling stability and capacitive performance. J. Mater. Sci.: Mater. Electron. 34(36), 2308 (2023)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Materials preparation, Data curation, Investigation, Methodology, Formal analysis, Writing-Original draft was done by AP. The experiments and results were validated by AS, AB, JS, SP, KA, and SS. The research work conceptualization, formal analysis, funding acquisition, project administration, resources, supervision, and writing—review & editing were done by MS and SS. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
All the authors declare that this research article is original and have not been in the consideration in other journals or publications. Also, authors assure that there are no ethical violations in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Priyadharsini, A., Saravanakumar, M., Sakunthala, A. et al. Role of preparation conditions on the pseudocapacitor properties of SnO2 nanoparticles by co-precipitation method. J Mater Sci: Mater Electron 35, 451 (2024). https://doi.org/10.1007/s10854-024-12239-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12239-7