Abstract
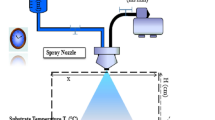
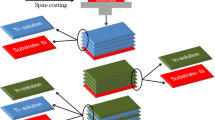
In present study, ZnO/SnO2/ZnO/SnO2/ZnO multi–layer, ZnO/SnO2/ZnO triple layer and ZnO single layer films have been deposited on glass substrate by sol–gel dip–coating technique. The structural and optical properties of thin films have been investigated by X-ray diffractometer, UV–visible, photoluminescence spectroscopies and scanning electron microscopy. The structural analysis reveals structural inhomogeneities and different crystallite growth processes as function of number of deposited layers. A comparison between photocatalytic activity of zinc oxide samples toward photodegradation of phenol, 4-aminophenol and 4-nitrophenol has been performed under UV light irradiation. Experiments were conducted to study the effects of operational parameters on the degradation rate. Pseudo-first-order photodegradation kinetics was observed on all films and the reaction constants were determined. The results showed that the photocatalytic activity of ZnO multi–layer film was superior to that of the ZnO single- and triple-layer films. Differences in film efficiencies can be attributed to differences in crystallinity, surface morphology, defect concentration of oxygen vacancy and to presence of SnO2 sublayer that may act as trap for electrons generated in the ZnO layer thus preventing electron–hole recombination. The results reveal that SnO2 hetrojunction layers improve crystalline quality, optical and photocatalytic properties of ZnO multilayered films.











Similar content being viewed by others
References
Lathasree S, Rao AN, SivaSankar B, Sadasivam V, Rengaraj K (2004) Heterogeneous photocatalytic mineralisation of phenols in aqueous solutions. J Mol Catal A: Chem 223:101–104
Buikema AL Jr, McGinniss MJ, Caims J Jr (1979) Phenolics in aquatic ecosystems: a selected review of recent literature. Mar Environ Res 2:87–181
American Conference of Governmental Industrial Hygienists Standards (ACGIH) Manual (2005) and United States Environmental Protection Agency, EPA, 816-F-01-007, 2006
Busca G, Berardinelli S, Resini C, Arrighi L (2008) Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater 160:265–288
Huang A et al (1999) Photocatalytic degradation of triethylamine on titanium oxide thin films. J Catal 188:40–45
San N, Hatipoglu A, Kocturk G, Cinar Z (2002) Photocatalytic degradation of 4-nitrophenol in aqueous TiO2 suspensions: theoretical prediction of the intermediates. J Photochem Photobiol A chem 146:189–197
Dieckmann MS, Gray KA (1996) A comparison of the degradation of 4-nitrophenol via direct and sensitized photocatalysis in TiO2 slurries. Water Res 30:1169–1183
Chen D, Ray AK (1998) Photodegradation kinetics of 4-nitrophenol in TiO2 suspension. Water Res 32:3223–3228
Park Y, Skelland AHP, Forney LJ, Kim JH (2006) Removal of phenol and substituted phenols by newly developed emulsion liquid membrane process. Water Res 40:1763–1772
Maurino V, Minero C, Pelizzetti E, Piccinini P, Serpone N, Hidaka H (1997) The fate of organic nitrogen under photocatalytic conditions: degradation of nitrophenols and aminophenols on irradiated TiO2. J Photochem Photobiol A Chem 109:171–179
Khan SA, Hamayun M, Ahmed S (2006) Degradation of 4-aminophenol by newly isolated Pseudomonas sp. strain ST-4. Enzyme Microb Technol 38:10–19
He Z, Song S, Ying H, Xu L, Chen J (2007) p-Aminophenol degradation by ozonation combined with sonolysis: operating conditions influence and mechanism. Ultrason Sonochem 14:568–574
Karunakaran C, Dhanalakshmi R (2008) Semiconductor-catalyzed degradation of phenols with sunlight. Sol Energy Mater Sol Cells 92:1315–1321
Gouvea K, Wypych F, Moraes SG, Duran N, Nagata N, Peralta-Zamora P (2000) Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere 40:433–441
Dindar S, Icli J (2001) Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J Photochem Photobiol A chem 140:263–276
Sakthivel S, Neppolian B, Shankar MV, Arabindoo B, Palanichamy M, Murugesan V (2003) Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO2. Sol Energy Mater Sol Cells 77:65–82
Khodja AA, Sehili T, Pilichowski JF, Boule P (2001) Photocatalytic degradation of 2-Phenyphenol on TiO2 and ZnO in aqueous suspensions. J Photochem Photobiol A Chem 141:231–239
Lizama C, Freer J, Baeza J, Mansilla HD (2002) Optimized photodegradation of reactive blue 19 on TiO2 and ZnO suspensions. Catal Today 76:235–249
Wang J et al (2009) Sonocatalytic degradation of some dyestuffs and comparison of catalytic activities of nano-sized TiO2, nano-sized ZnO and composite TiO2/ZnO powders under ultrasonic irradiation. Ultrason Sonochem 16:225–231
Chakrabarti S, Chaudhuri B, Bhattacharjee S, Das P, Dutta BK (2008) Degradation mechanism and kinetic model for photocatalytic oxidation of PVC–ZnO composite film in presence of a sensitizing dye and UV radiation. J Hazard Mater 154:230–236
Pal B, Sharon M (2002) Enhanced photocatalytic activity of highly porous ZnO thin films prepared by sol–gel process. Mater Chem Phys 76:82–87
Wang Y, Li X, Wang N, Quan X, Chen Y (2008) Controllable synthesis of ZnO nanoflowers and their morphology-dependent photocatalytic activities. Sep Purif Technol 62:729–734
Suárez-Parra R, Hernández-Pérez I, Rincón ME, López-Ayala S, Roldán-Ahumad MC (2003) Visible light-induced degradation of blue textile azo dye on TiO2/CdO–ZnO coupled nanoporous films. Sol Energy Mater Sol Cells 76:189–199
Park DJ, Lee JY, Park TE, Kim YY, Cho HK (2007) Improved microstructural properties of a ZnO thin film using a buffer layer in situ annealed in argon ambient. Thin Solid Films 515:6721–6726
Koike K, Komuro T, Ogata K, Sasa S, Inoue M, Yano M (2004) CaF2 growth as a buffer layer of ZnO/Si heteroepitaxy. Phys E 21:679–683
Fujita M, Sasajima M, Deesirapipat Y, Horikoshi Y (2005) Molecular beam epitaxial growth of hexagonal ZnMgO films on Si(111) substrates using thin MgO buffer layer. J Cryst Growth 278:293–298
Zhang Y, Zheng H, Su J, Lin B, Fu Z (2007) Effects of SiC buffer layer on the optical properties of ZnO films grown on Si (111) substrates. J Lumin 124:252–256
Li F et al (2006) Effect of the initial thin Ti buffer layers on the quality of ZnO thin films grown on Si(111) substrates by MOCVD. Superlatt Microstruct 40:56–63
Xu L, Shi L, Li X (2008) Effect of TiO2 buffer layer on the structural and optical properties of ZnO thin films deposited by E-beam evaporation and sol–gel method. Appl Surf Sci 255:3230–3234
Choi WS, Kim EJ, Seong S, Kim YS, Park C, Hahn SH (2009) Optical and structural properties of ZnO/TiO2/ZnO multi-layers prepared via electron beam evaporation. Vacuum 83:878–882
Vinodkumar R et al (2010) Effect of ITO buffer layers on the structural, optical and electrical properties of ZnO multilayer thin films prepared by pulsed laser deposition technique. Sol Energy Mater Sol Cells 94:68–76
Summi R, Marley JA, Boncelli NF (1984) The ultraviolet absorption edge of stannic oxide (SnO2). J Phys Chem Solids 25:1465–1469
Cheng C et al (2009) Hierarchical assembly of ZnO nanostructures on SnO2 backbone nanowires: low temperature hydrothermal preparation and optical properties. J ACS Nano 3:3069–3076
Wang WW, Zhu YJ, Yang LX (2007) ZnO–SnO2 hollow spheres and hierarchical nanosheets: hydrothermal preparation, formation mechanism, and photocatalytic properties. Adv Funct Mater 17:59–64
Agnihotri OP, Mohammad MT, Abass AK, Arshak KI (1983) Electrical and optical properties of chemically deposited conducting glass for SIS solar cells. Solid State Commun 47:195–203
Hong R, Shao J, He H, Fan Z (2006) Enhancement of near band edge photoluminescence of ZnO thin films in sandwich configuration at room temperature. J Appl Phys 99:093520–093523
Herrero J, Guillen C (2004) Improved ITO thin films for photovoltaic applications with a thin ZnO layer by sputtering. Thin Solid Films 451:630–633
Wang XT, Zhong SH, Xiao XF (2005) Photo-catalysis of ethane and carbon dioxide to produce hydrocarbon oxygenates over ZnO-TiO2/SiO2 catalyst. J Mol Catal A: Chem 229:87–93
Shouqiang W, Zhongcai S, Xudong L, Ying L, Linlin C, Yan H (2009) Photocatalytic degradation of methyl orange over ITO/CdS/ZnO interface composite films. J Environ Sci 21:991–996
Lee DN (2003) Elastic properties of thin films of cubic system. Thin Solid Films 434:183–189
Li F, Ding Y, Gao P, Xin X, Wang Z (2004) Single-crystal hexagonal disks and rings of zno: low-temperature, large-scale synthesis and growth mechanism. Angew Chem Int Ed 43:5238–5246
Cho S, Jung SH, Lee KH (2008) Morphology-controlled growth of ZnO nanostructures using microwave irradiation: from basic to complex structures. J Phys Chem C 112:12769–12776
Vayssieres L, Keis K, Hagfeldt A, Lindquist SE (2001) Three-dimensional array of highly oriented crystalline ZnO microtubes. Chem Mater 13:4395–4398
Ghosh R, Basak D, Fujihara S (2004) Effect of substrate-induced strain on the structural, electrical, and optical properties of polycrystalline ZnO thin films. J Appl Phys 96:2689–2697
Selected Powder Diffraction Data for Metals and Alloys (1978) vol 1. JCPDS, USA, pp 108
Zhang Y, Lin B, Fu Z, Liu C, Han W (2006) Strong ultraviolet emission and rectifying behavior of nanocrystalline ZnO films. Opt Mater 28:1192–1196
Bao D, Gu H, Kuang A (1998) Sol–gel-derived c-axis oriented ZnO thin films. Thin Solid Films 312:37–39
Fernandez T, Jose G, Mathew S, Rejikumar PR, Unnikrishnan NV (2007) An ultra- low hydrolysis sol–gel route for titanosilicate xerogels and their characterization. J Sol–Gel Sci Technol 41:163–168
Tan ST et al (2005) Blue shift of optical band gap in ZnO thin films grown by metal-organic chemical vapour deposition. J Appl Phys 98:013505–013509
Qadri SB, Yang JP, Skelton EF, Ratna BR (1997) Evidence of strain and lattice distortion in lead sulphide nanocrystallites. Appl Phys Lett 70:1020–1021
Cullity BD (1978) Elements of X-ray diffractions. Addison-Wesley, Reading, p 102
Williamson GK, Hall H (1953) X-ray line broadening from filed aluminiumand wolfram. Acta Metall 1:22–31
Sakthivel S, Geissen U, Bahnemann DW (2002) Enhancement of photocatalytic activity by semiconductor heterojunctions: α-Fe2O3, WO3 and CdS deposited on ZnO. J Photochem Photobiol A Chem 148:283–293
Frank G, Kauer E, Kostlin H (1981) Transparent heat-reflecting coatings based on highlydoped semieonduetors. Thin Solid Films 77:107–118
Paras N (2004) Nanophotonics. Wiley Interscience, New Jersey, p 34
Efros AL (1982) Interband absorption of light in a semiconductor sphere. Sov Phys Semicond 16:772–775
Pankove JI (1971) Optical processes in semiconductors, chapter 2. Dover Publication, New York, p 22
Zhou WD, Wu X, Zhang YC (2007) Solvothermal synthesis of hexagonal ZnO nanorods and their photoluminescence properties. Mater Lett 61:2054–2059
Kar S, Dev A, Chaudhuri S (2006) simple solvothermal route to synthesize zno nanosheets, nanonails, and well-aligned nanorod arrays. J Phys Chem B 110:17848–17857
Zhang YC, Wu X, Hu XY (2005) Low-temperature synthesis of nanocrystalline ZnO by thermal decomposition of a “green” single-source inorganic precursor in air. J Cryst Growth 280:250–254
Pal U, Santiago P (2005) Controlling the morphology of ZnO nanostructures in a low-temperature hydrothermal process. J Phys Chem B 109:15317–15321
Kar S, Pal BN, Chaudhuri S, Chakravorty D (2006) One-dimensional ZnO nanostructure arrays:# synthesis and characterization. J Phys Chem B 110:4605–4611
Mahamuni S, Borgohain K, Bendre BS, Leppert VJ, Risbud SH (1999) Spectroscopic characterization of electrochemically grown ZnO quantum dots. J Appl Phys 85:2861–2868
Sagar P, Shishodia PK, Mehra RM, Okada H, Wakahara A, Yoshida A (2007) Photoluminescence and absorption in sol–gel-derived ZnO films. J Lumin 126:800–806
Sun X, Qiu X, Li L, Li G (2008) ZnO twin-cones: synthesis, photoluminescence, and catalytic decomposition of ammonium perchlorate. Inorg Chem 47:4146–4152
Zheng Y et al (2007) Ag/ZnO heterostructure nanocrystals: synthesis, characterization and photocatalysis. Inorg Chem 46:6675–6682
Vanheusden K, Warren WL, Seager CH, Tallant DR, Voigt JA, Gnade BE (1996) Mechanisms behind green photoluminescence in ZnO phosphor powders. J Appl Phys 79:7983–7990
Jing LQ et al (2006) Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol Energy Mater Sol Cells 90:1773–1787
Xu Y, Schoonen MAA (2000) The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am Miner 85:543–556
Li GR et al (2008) Morphology—function relationship of ZnO: polar planes, oxygen vacancies, and activity. J Phys Chem C 112:11859–11864
Zhang J, Sasaki K, Sutter E, Adzic RR (2007) Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 315:220–227
Paz Y, Luo Z, Rabenberg L, Heller A (1995) Photooxidative self-cleaning transparent titanium dioxide films on glass. J Mater Res 10:842–2848
Yu J, Zhao X (2000) Effect of substrates on the photocatalytic activity of nanometer TiO2 thin films. Mater Res Bull 35:1293–1301
Ahmed S, Rasul MG, Wayde N, Martens R, Brown MA, Hashib S (2010) Heterogeneous photocatalytic degradation of phenols in wastewater: a review on current status and developments. Desalination 261: 3–18 and the references cited in there
Brezova V, Ceppan M, Brandsteterova E, Breza M, Lapcik L (1991) Photocatalytic hydroxylation of benzoic acid in aqueous titanium dioxide suspension. J Photochem Photobiol A 59:385–391
Eberhardt MK, Yoshida M (1973) Radiation-induced homolytic aromatic substitution. I. Hydroxylation of nitrobenzene, chlorobenzene, and toluene. J Phys Chem 77:589–597
Acknowledgments
The authors are grateful for the financial support provided by Islamic Azad University, Shahreza branch.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talebian, N., Nilforoushan, M.R. & Memarnezhad, P. Photocatalytic activities of multilayered ZnO-based thin films prepared by sol–gel route: effect of SnO2 heterojunction layer. J Sol-Gel Sci Technol 65, 178–188 (2013). https://doi.org/10.1007/s10971-012-2922-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2922-4