Abstract
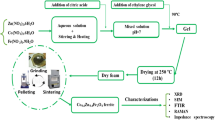
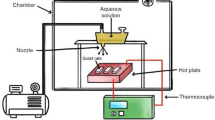
Ca0.6La0.267TiO3 nanocrystalline powders were successfully synthesized by the sol–gel method using PEG1000 as a dispersant in this study. The sinterability of the powders and the microwave dielectric properties of the ceramics were also investigated. The XRD diffraction result showed that pure Ca0.6La0.267TiO3 powder with orthorhombic perovskite structure could be synthesized at 600 °C for 2 h without any detectable intermediate phase. The average grain size of the as-synthesized powder was as low as 35 nm. Compared with Ca0.6La0.267TiO3 ceramics fabricated by conventional solid-state process, the bulk materials prepared by sintering as-prepared nanopowders performed better in densification and microwave dielectric properties. The ceramics sintered at 1,300 °C exhibited a higher relative density of 98.3% combined with a dielectric constant (ε r ) of 120.3, a quality factor (Q × f) of 23,550 GHz and a temperature coefficient of resonant frequency (τ f ) of +220.7 ppm/°C, respectively.








Similar content being viewed by others
References
Kolar D, Stadler Z, Gaberscek S, Suvorov D (1978) Ber Dtsch Keram Ges 55:346–348
Takahashi H, Baba Y, Ezaki K, Okamoto Y, Shibata K, Kuroki K, Nakano S (1991) Jpn J Appl Phys 30:2339–2342
Yoshida M, Hara N, Takada T, Seki A (1997) Jpn J Appl Phys 36:6818–6823
Kell RC, Greenham AC, Olds GCE (1973) J Am Ceram Soc 56:352–354
Jancar B, Suvorov D, Valant M, Drazic G (2003) J Eur Ceram Soc 23:1391–1400
Ezaki K, Baba Y, Takahashi H, Shibata K, Nakano S (1993) Jpn J Appl Phys 32:4319–4322
Kim ES, Yoon KH (2003) J Eur Ceram Soc 23:2397–2401
Liu T, Zhao XZ (2006) J Am Ceram Soc 89:1153–1155
Kim IS, Jung WH, Inaguma Y, Nakamura T, Itoh M (1995) Mater Res Bull 30:307–316
Huang CL, Tsai JT, Chen YB (2001) Mater Res Bull 36:547–556
Kim WS, Kim ES, Yoon KH (1999) J Am Ceram Soc 82:2111–2115
Wang H, Chen W, Liu T (2004) J Ceram 25:47–51 (in Chinese)
Huang CL, Liu SS (2008) Mater Lett 62:3205–3208
Huang CL, Pan CL, Hsu JF, Wang JJ (2008) J Alloys Compd 461:521–526
Chen YB (2009) J Alloys Compd 480:820–823
Han KR, Jang JW, Cho SY, Jeong DY, Hong KS (1998) J Am Ceram Soc 811:209–1214
Li W, Zhuo MW, Shi JL (2004) Mater Lett 58:365–368
Xu YB, Huang GH, He YY (2005) Ceram Int 31:21–25
Vidyasagar K, Gopalkrishnan J, Rao CNR (1985) J Solid State Chem 58:29–37
Padmini P, Kutty TR (1994) J Mater Chem 4:1875–1882
Kumar MD, Srinivasan TM, Ramasamy P, Subramanian C (1995) Mater Lett 25:171–174
Douy A, Capron M (2003) J Eur Ceram Soc 23:2075–2081
Stöber W, Fink A, Bohn E (1968) J Colloid Interface Sci 26:62–69
Livage J (1999) Bull Mater Sci 22:201–205
Roy R (1987) Science 238:664–1669
Behera SK, Sahu PK, Pratihar SK, Bhattacharyya S (2004) Mater Lett 58:3710–3715
Meiszterics A, Sinkó K (2008) Colloids Surf A 319:143–148
Yu PF, Wang X, Cui B (2007) Scripta Mater 57:623–626
Zhang QL, Wu F, Yang H, Zou D (2008) J Mater Chem 18:5339–5343
Hakki B, Coleman PD (1960) IRE Trans Microw Theory Tech MTT-8:402–410
Courtney WE (1970) IRE Trans Microw Theory Tech MTT-18:476–485
Wang HP, Zhang QL, Yang H, Sun HP (2007) Acta Phys Chim Sin 23:609–613 (in Chinese)
Warren BE (1969) X-ray diffraction. Addison-Wesley, Reading
Arlt G, Hennings DFK, With GD (1985) J Appl Phys 58:1619–1625
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Qiu, T., Fan, C. et al. Synthesis and microwave dielectric properties of Ca0.6La0.267TiO3 nanocrystalline powders by sol–gel method. J Sol-Gel Sci Technol 59, 525–531 (2011). https://doi.org/10.1007/s10971-011-2522-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2522-8