Abstract
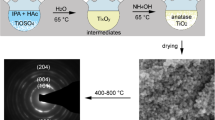
Mesoporous titania nanoparticles (denoted as MTN) with high surface area (e.g., 252 m2 g−1) were prepared using tetrapropyl orthotitanate (TPOT) as a titania precursor and 10–20 nm or 20–30 nm silica colloids as templates. Co-assembly of TPOT and silica colloids in an aerosol-assisted process and immediate calcination at 450 °C resulted in anatase/silica composite nanoparticles. Subsequent removal of the silica colloids from the composite by NaOH solution created mesopores in the TiO2 nanoparticles with pore size corresponding to that of silica colloids. Effects of silica colloids’ contents on MTN porosity and crystallites’ growth at a higher calcination temperature (e.g., 1000 °C) were investigated. Silica colloids suppressed the growth of TiO2 crystallites during calcination at a higher calcination temperature and controllable contents of the silica colloids in precursor solution resulted in various atomic ratios of anatase to rutile in the calcinated materials. The mesostructure and crystalline structure of these titania materials were characterized by transmission electron microscope (TEM), scanning electron microscope (SEM), X-ray diffraction (XRD), differential thermal analysis (DTA)-thermo-gravimetric analysis (TGA), and N2 sorption.







Similar content being viewed by others
References
Khan SUM, Shahry MA, Ingler WB (2002) Science 297:2243
Stiehl JD, Kim TS, McClure SM, Mullins CB (2004) J Am Chem Soc 126:13574
Yu JC, Zhang LC, Yu JG (2002) Chem Mater 14:4647
Cabrera S, El-Haskouri J, Beltran-Portier A, Beltran-Portier D, Marcos AD, Amoros P (2000) Solid State Sci 2:513
Wagemaker M, Kentgens APM, Mulder FM (2002) Nature 418:397
Chen MS, Goodman DW (2004) Science 306:252
Gao XP, Zhu HY, Pan GL, Ye SH, Lan Y, Wu F, Song DY (2004) J Phys Chem B 108:2868
Ovenstone J, Yanagisawa K (1999) Chem Mater 11:2770
Kavan L, Kalbac M, Zukalova M, Exnar I, Lorenzen V, Nesper R, Graetzel M (2004) Chem Mater 16:477
Stone VF, Davis RJ (1998) Chem Mater 10:1468
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Nature 359:710
Attard GS, Glyde JC, Goltner CG (1995) Nature 378:366
Davis SA, Burkett SL, Mendelson NH, Mann S (1997) Nature 385:420
Huo Q, Leon R, Petroff PM, Stucky GD (1995) Science 268:1324
Li D, Zhou H, Honma I (2004) Nat Mater 3:65
Miyata H, Suzuki T, Fukuoka A, Sawada T, Watanabe M, Noma T, Takada K, Mukaide T, Kuroda K (2004) Nat Mater 3:651
Shibata H, Ogura T, Mukai T, Ohkubo T, Sakai H, Abe M (2005) J Am Chem Soc 127:16396
Peng T, Zhao D, Dai K, Shi W, Hirao K (2005) J Phys Chem B 109:4947
Liu H, Yang W, Ma Y, Ye X, Yao J (2003) New J Chem 27:529
Wu ZW, Hu QY, Pang JB, Jakobsen HP, Yu DH, Lu YF (2005) Microporous Mesoporous Mater 85:305
Hampsey JE, Hu Q, Wu Z, Rice L, Pang J, Lu Y (2005) Carbon 43:2977
Hampsey JE, Hu Q, Rice L, Pang J, Wu Z, Lu Y (2005) Chem Commun 28:3606
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity, 2nd edn. Academic Press, London
Huang W, Tang X, Wang Y, Koltypin Y, Gedanken A (2000) Chem Commun 1415
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1998) Langmuir 14:3160
Acknowledgments
The authors gratefully acknowledge the financial support of this work by NASA (Grant No. NAG-1-02070 and NCC-3-946), the Office of Naval Research, the Louisiana Board of Regents (Grant No. LEQSF(2001-04)-RD-B-09), National Science Foundation (Grant No. NSF-DMR-0124765, and CAREER award).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, Z., Lu, Y. Aerosol-assisted synthesis of mesoporous titania nanoparticles with high surface area and controllable phase composition. J Sol-Gel Sci Technol 53, 287–292 (2010). https://doi.org/10.1007/s10971-009-2089-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-2089-9