Abstract
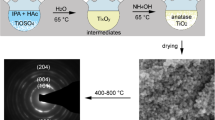
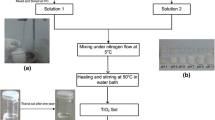
Highly homogeneous transparent titania gels have been successfully prepared from titanium alkoxide by a sol–gel method utilizing chelating agent, ethyl acetylacetate (EtAcAc), in the presence of strong acid anions. Only catalytic amount of a strong acid anion suppress the rapid hydrolysis of titanium alkoxide by blocking the nucleophilic attack of HO− and H2O, and the resultant moderate sol–gel reactions thus afford homogeneous gelation, leading to transparent monolithic titania gels. Gelation time can be widely controlled by changing amounts of water, chelating agent and salt. The ability of salts to suppress the too abrupt sol–gel reactions is strongly dependent on the electronegativity of anions and valence of cations. With employing NH4NO3 as a suppressing electrolyte, the obtained titania gels can be converted to pure TiO2 by simple washing and heat-treatment, and transformations to anatase and rutile structures were found to start at 400 and 600 °C, respectively.










Similar content being viewed by others
References
Anderson MA, Gieselmann MJ, Xu Q (1988) J Memb Sci 39:243–258
Kurganov A, Trüdinger U, Isaeva T, Unger K (1996) Chromatographia 42:217–222
Buchmeiser MR (2001) J Chromatogr A 918:233–266
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Science 293:269–271
Khan SUM, Al-Shahry M, Ingler WB (2002) Science 297:2243–2245
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Chem Rev 95:69–96
Fujishima A, Honda K (1972) Nature 238:37–38
O’Regan B, Gratzel M (1991) Nature 353:737–740
Carp O, Huisman CL, Reller A (2004) Progr Solid State Chem 32:33–177
Sakai N, Ebina Y, Takada K, Sasaki T (2004) J Am Chem Soc 126:5851–5858
Kavan L, Kalbac M, Zukalova M, Exnar I, Lorenzen V, Nesper R, Graetzel M (2004) Chem Mater 16:477–485
Kim HS, Gilmer DC, Campbell SA, Polla DL (1996) Appl Phys Lett 69:3860–3862
Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T (1997) Nature 388:431–432
Yu JC, Zhang L, Yu J (2002) Chem Mater 14:4647–4653
Hague DC, Mayo MJ (1994) J Am Ceram Soc 77:1957–1960
Negishi N, Iyoda T, Hashimoto K, Fujishima A (1995) Chem Lett 24:841–842
Matsuda A, Kotani Y, Kogure T, Tatsumisago M, Minami T (2000) J Am Ceram Soc 83:229–231
Kajihara K, Nakanishi K, Tanaka K, Hirao K, Soga N (1998) J Am Ceram Soc 81:2670–2676
Yoldas BE (1980) J Non-Cryst Solids 38:81–86
Mariscal R, Palacios JM, Galan-Fereres M, Fierro JLG (1994) Appl Catal A 116:205–219
Kim WI, Hong IK (2003) J Ind End Chem 9:728–734
Lee JH, Choi SY, Kim CE (1997) J Mater Sci 32:3577–3585
Yoldas BE (1986) J Mater Sci 21:1087–1092
Terabe K, Kato K, Miyazaki H, Yamaguchi S, Imai A, Iguchi Y (1994) J Mater Sci 29:1617–1622
Konishi J, Fujita K, Nakanishi K, Hirao K (2006) Chem Mater 18:6069–6074
Yao B, Zhang L (1999) J Mater Sci 34:5983–5987
Mir LE, Amlouk A, Elaloui E, Saadoun M, Pierre AC (2008) Mater Sci Eng B 146:69–73
Moriguchi I, Maeda H, Teraoka Y, Kagawa S (1997) Chem Mater 9:1050–1057
Arconada N, Durán A, Suárez S, Portela R, Coronado JM, Sánchez B, Castro Y (2009) Appl Catal B 86:1–7
Wellbrock U, Beier W, Frischat GH (1992) J Non-Cryst Solids 147:350–355
Matijević E, Scheiner P (1978) J Colloid Interface Sci 63:509–524
Attar AS, Ghamsari MS, Hajiesmaeilbaigi F, Mirdamadi S (2008) J Mater Sci 43:1723–1729
Yamada N, Yoshinaga I, Katayama S (2000) J Sol-Gel Sci Technol 17:123–130
Kallala M, Sanchez C, Cabane B (1993) Phys Rev E 48:3692–3704
Blanchard J, In M, Schaudel B, Sanchez C (1998) Eur J Inorg Chem 1115–1127
Matijević E, Budnik M, Meites L (1977) J Colloid Interface Sci 61:302–311
Noda LK, de Almeida RM, Probst LFD, Gonçalves NS (2005) J Mol Catal A 225:39–46
Minero C, Mariella G, Maurino V, Pelizzetti E (2000) Langmuir 16:2632–2641
Calza P, Pelizzeti E, Mogyorósi K, Kun R, Dékány I (2007) Appl Catal 72:314–321
Brinker CJ, Scherer GW (1990) Sol-Gel Science. Academic Press Inc, New York
Allred AL, Rochow EG (1958) J Inorg Nucl Chem 5:264–268
Acknowledgments
The Grant-in-Aid for Scientific Research (No. 20750177 for K. K. and No. 20350094 for K. N.) and the Global COE Program “International Center for Integrated Research and Advanced Education in Materials Science” (No. B-09) both from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, are acknowledged. Also, partly supported by a Grant for Practical Application of University R&D Results under the Matching Fund Method from New Energy and Industrial Technology Development Organization (NEDO), Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasegawa, G., Kanamori, K., Nakanishi, K. et al. Facile preparation of transparent monolithic titania gels utilizing a chelating ligand and mineral salts. J Sol-Gel Sci Technol 53, 59–66 (2010). https://doi.org/10.1007/s10971-009-2056-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-2056-5