Abstract
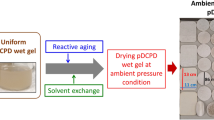
Less fragile lightweight nanostructured polyurea based organic aerogels were prepared via a simple sol–gel processing and supercritical drying method. The uniform polyurea wet gels were first prepared at room temperature and atmospheric pressure by reacting different isocyanates with polyamines using a tertiary amine (triethylamine) catalyst. Gelation kinetics, uniformity of wet gel, and properties of aerogel products were significantly affected by both target density (i.e., solid content) and equivalent weight (EW) ratio of the isocyanate resin and polyamine hardener. A supercritical carbon dioxide (CO2) drying method was used to extract solvent from wet polyurea gels to afford nanoporous aerogels. The thermal conductivity values of polyurea based aerogel were measured at pressures from ambient to 0.075 torr and at temperatures from room temperature to −120 °C under a pressure of 8 torr. The polyurea based aerogel samples demonstrated high porosities, low thermal conductivity values, hydrophobicity properties, relatively high thermal decomposition temperature (~270 °C) and low degassing property and were less dusty than silica aerogels. We found that the low thermal conductivities of polyurea based aerogels were associated with their small pore sizes. These polyurea based aerogels are very promising candidates for cryogenic insulation applications and as a thermal insulation component of spacesuits.













Similar content being viewed by others
References
Kistler SS (1931) Nature 127:741. doi:10.1038/127741a0
Kistler SS (1932) J Phys Chem 36:52. doi:10.1021/j150331a003
Hüsing N, Schubert U (1998) Angew Chem Int Ed 37:22
Pierre AC, Pajonk GM (2002) Chem Rev 102:4243. doi:10.1021/cr0101306
Pajonk GM (2003) Colloid Polym Sci 281:637. doi:10.1007/s00396-002-0814-9
Bisson A, Rigacci A, Lecomte D, Rodier E, Achard P (2003) Dry Technol 21:593. doi:10.1081/DRT-120019055
Akimov YK (2003) Instrum Exp Tech 46:287. doi:10.1023/A:1024401803057
LeMay JD, Hopper RW, Hrubesh LW, Pekala RW (1990) MRS Bull 15(12):19
Schaefer D (1994) MRS Bull 19(4):49
Hrubesh LW, Poco JF (1995) J Non-Cryst Solids 188:46. doi:10.1016/0022-3093(95)00028-3
Schmidt M, Schwertfeger F (1998) J Non-Cryst Solids 225:364. doi:10.1016/S0022-3093(98)00054-4
Fricke J, Emmerling A (1998) J Sol–Gel Sci Technol (Paris) 13:299
Pekala RW, Schaefer DW (1993) Macromolecules 26:5487. doi:10.1021/ma00072a029
Pekala RW (1989) J Mater Sci 24:3221. doi:10.1007/BF01139044
Pekala RW, Kong FM (1989) Polymer Prepr 30:221
Ward RL, Pekala RW (1990) Polymer Prepr 31:167
Pekala RW, Alviso CT, LeMay JD (1990) J Non-Cryst Solids 125:67. doi:10.1016/0022-3093(90)90324-F
Pekala RW, Alviso CT, Kong FM, Hulsey SS (1992) J Non-Cryst Solids 145:90. doi:10.1016/S0022-3093(05)80436-3
Lu X, Arduini-Schuster MC, Kuhn J, Nilsson O, Fricke J, Pekala RW (1992) Science 255:971. doi:10.1126/science.255.5047.971
Lu X, Caps R, Fricke J, Alviso CT, Pekala RW (1995) J Non-Cryst Solids 188:226. doi:10.1016/0022-3093(95)00191-3
Tan C, Fung BM, Newman JK, Vu C (2001) Adv Mater 13:644. doi:10.1002/1521-4095(200105)13:9<644::AID-ADMA644>3.0.CO;2-#
Fischer F, Rigarcci A, Pirad R, Berthon-Fabry S, Achard P (2006) Polymer (Guildf) 47:7636. doi:10.1016/j.polymer.2006.09.004
Biesmans G, Randall D, Francais E, Perrut M (1998) J Non-Cryst Solids 225:36. doi:10.1016/S0022-3093(98)00103-3
Biesmans G, Mertens A, Duffours L, Woignier T, Phalippou J (1998) J Non-Cryst Solids 225:64. doi:10.1016/S0022-3093(98)00010-6
Lee JK, Shannon W, Mesham M, Gould GL (2006) NASA SBIR Phase II Contract No. NNJ04JA22C Final Report, March
Rigacci A, Marechal JC, Repoux M, Moreno M, Achard P (2004) J Non-Cryst Solids 350:372. doi:10.1016/j.jnoncrysol.2004.06.049
Lee JK, Gould GL (2007) J Sol–Gel Sci Technol (Paris) 44:29
Kramer DP, McNeil DC, Howell EI, Gembarovic J, Taylor RE (2002) Proceedings of the 37th intersociety energy conversion engineering conference (IECEC), IEEE, Catalog #02CH37298, July 2002, paper 20089
Bittle RR, Taylor RE (1984) J Am Ceram Soc 67:186
Lee JK, Gould GL (2005) J Sol–Gel Sci Technol (Paris) 34:281
Hummer E, Rettelbach T, Lu X, Fricke J (1993) Thermochim Acta 218:269. doi:10.1016/0040-6031(93)80428-D
Lee OJ, Lee KH, Kim SY, Yoo KP (2002) J Non-Cryst Solids 298:287. doi:10.1016/S0022-3093(01)01041-9
Scheuerpflug P, Morper HJ, Neubert G, Fricke J (1991) J Phys D Appl Phys 24:1395. doi:10.1088/0022-3727/24/8/025
Acknowledgment
This work was conducted by the financial support of the United States National Aeronautics and Space Administration (NASA), SBIR Contract No. NNJ04JA22C. The authors are grateful to Mr. Max Mesham, Ms. Geeta Bhakhari, and Mr. Nathan Bhobho for their helps in preparing samples and also, to Ms. Sara Rosenberg, Dr. Jenifer Marchesi, and Dr. Shannon White for their valuable helps. The authors would like to thank Ms. Evelyn S. Orndoff and Mr. Luis A. Trevino of NASA for their continuous supports for this work. The authors are also grateful to TPRL for thermal conductivity measurement at different pressures and temperatures and Dow Corning Analytical Lab for SEM measurement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.K., Gould, G.L. & Rhine, W. Polyurea based aerogel for a high performance thermal insulation material. J Sol-Gel Sci Technol 49, 209–220 (2009). https://doi.org/10.1007/s10971-008-1861-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-008-1861-6